Input interpretation
hydrogen cyanide | iodic acid
Chemical names and formulas
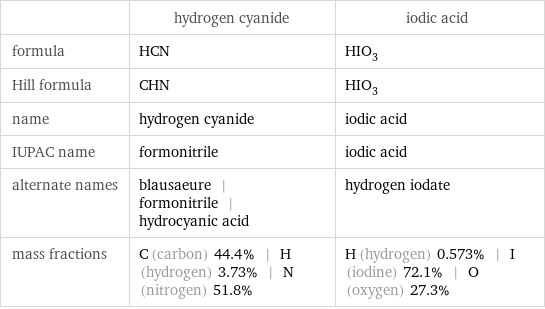
| hydrogen cyanide | iodic acid formula | HCN | HIO_3 Hill formula | CHN | HIO_3 name | hydrogen cyanide | iodic acid IUPAC name | formonitrile | iodic acid alternate names | blausaeure | formonitrile | hydrocyanic acid | hydrogen iodate mass fractions | C (carbon) 44.4% | H (hydrogen) 3.73% | N (nitrogen) 51.8% | H (hydrogen) 0.573% | I (iodine) 72.1% | O (oxygen) 27.3%
Structure diagrams
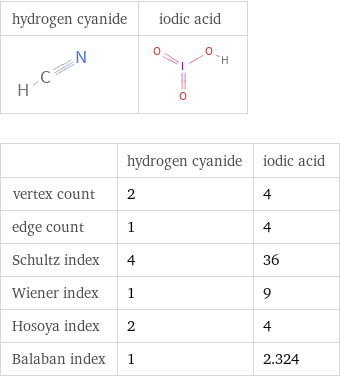
| hydrogen cyanide | iodic acid vertex count | 2 | 4 edge count | 1 | 4 Schultz index | 4 | 36 Wiener index | 1 | 9 Hosoya index | 2 | 4 Balaban index | 1 | 2.324
3D structure
3D structure
Basic properties
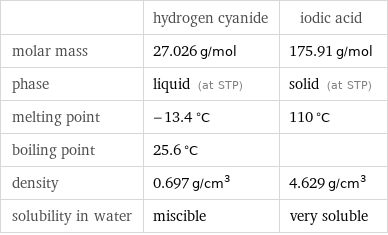
| hydrogen cyanide | iodic acid molar mass | 27.026 g/mol | 175.91 g/mol phase | liquid (at STP) | solid (at STP) melting point | -13.4 °C | 110 °C boiling point | 25.6 °C | density | 0.697 g/cm^3 | 4.629 g/cm^3 solubility in water | miscible | very soluble
Units

Liquid properties
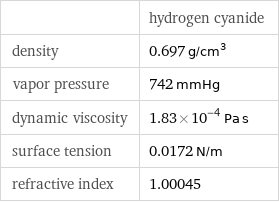
| hydrogen cyanide density | 0.697 g/cm^3 vapor pressure | 742 mmHg dynamic viscosity | 1.83×10^-4 Pa s surface tension | 0.0172 N/m refractive index | 1.00045
Units
Thermodynamic properties
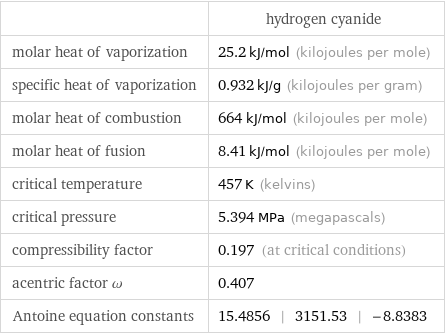
| hydrogen cyanide molar heat of vaporization | 25.2 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.932 kJ/g (kilojoules per gram) molar heat of combustion | 664 kJ/mol (kilojoules per mole) molar heat of fusion | 8.41 kJ/mol (kilojoules per mole) critical temperature | 457 K (kelvins) critical pressure | 5.394 MPa (megapascals) compressibility factor | 0.197 (at critical conditions) acentric factor ω | 0.407 Antoine equation constants | 15.4856 | 3151.53 | -8.8383
Solid properties
| iodic acid density | 4.629 g/cm^3 refractive index | 1.95
Units
