Input interpretation

fluorine (chemical element) | radon (chemical element) | nitrogen (chemical element)
Periodic table location
Periodic table location
Images

Images
Basic elemental properties
![| fluorine | radon | nitrogen atomic symbol | F | Rn | N atomic number | 9 | 86 | 7 short electronic configuration | [He]2s^22p^5 | [Xe]6s^24f^145d^106p^6 | [He]2s^22p^3 Aufbau diagram | 2p 2s | 6p 5d 4f 6s | 2p 2s block | p | p | p group | 17 | 18 | 15 period | 2 | 6 | 2 atomic mass | 18.998403163 u | 222 u | 14.007 u half-life | (stable) | 91.7639 h | (stable)](../image_source/774263b19cbcfb3926fcfc54c6c56714.png)
| fluorine | radon | nitrogen atomic symbol | F | Rn | N atomic number | 9 | 86 | 7 short electronic configuration | [He]2s^22p^5 | [Xe]6s^24f^145d^106p^6 | [He]2s^22p^3 Aufbau diagram | 2p 2s | 6p 5d 4f 6s | 2p 2s block | p | p | p group | 17 | 18 | 15 period | 2 | 6 | 2 atomic mass | 18.998403163 u | 222 u | 14.007 u half-life | (stable) | 91.7639 h | (stable)
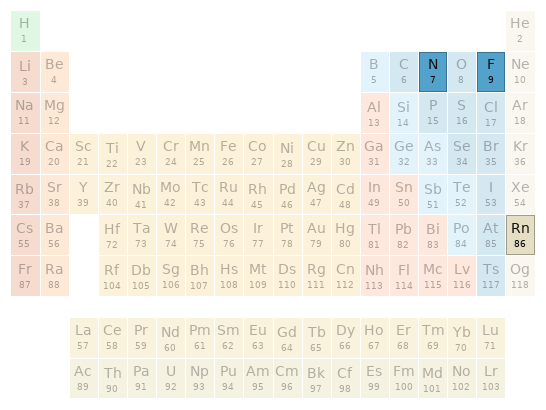
Thermodynamic properties
| fluorine | radon | nitrogen phase at STP | gas | gas | gas melting point | -219.6 °C | -71 °C | -210.1 °C boiling point | -188.12 °C | -61.7 °C | -195.79 °C critical temperature | 144.13 K | 377 K | 126.21 K critical pressure | 5.172 MPa | 6.28 MPa | 3.39 MPa molar heat of fusion | 0.26 kJ/mol | 3 kJ/mol | 0.36 kJ/mol molar heat of vaporization | 3.27 kJ/mol | 17 kJ/mol | 2.79 kJ/mol specific heat at STP | 824 J/(kg K) | 93.65 J/(kg K) | 1040 J/(kg K) adiabatic index | 7/5 | 5/3 | 7/5 (properties at standard conditions)
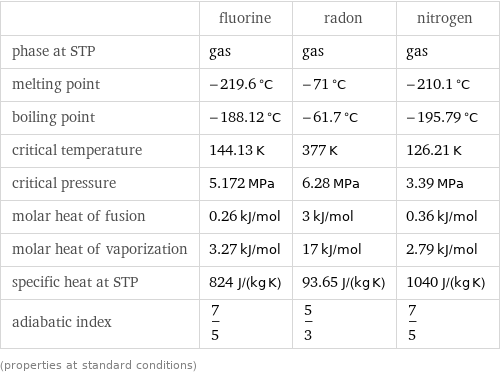
Material properties
| fluorine | radon | nitrogen density | 0.001696 g/cm^3 | 0.00973 g/cm^3 | 0.001251 g/cm^3 molar volume | 11200 cm^3/mol | 22800 cm^3/mol | 11200 cm^3/mol refractive index | 1.000195 | | 1.000298 sound speed | | | 333.6 m/s thermal conductivity | 0.0277 W/(m K) | 0.00361 W/(m K) | 0.02583 W/(m K) (properties at standard conditions)
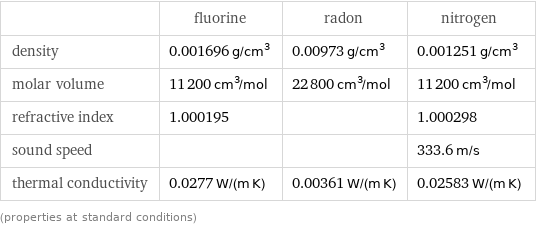
Electromagnetic properties
| fluorine | radon | nitrogen magnetic type | | | diamagnetic volume magnetic susceptibility | | | -6.8×10^-9 mass magnetic susceptibility | | | -5.4×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | | | -1.5×10^-10 m^3/mol (cubic meters per mole) color | (colorless) | (colorless) | (colorless) refractive index | 1.000195 | | 1.000298
Reactivity
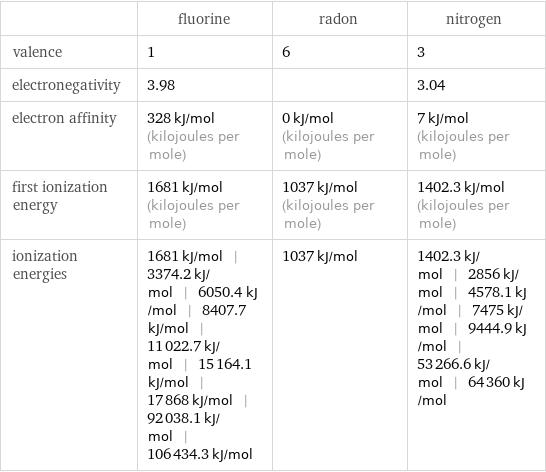
| fluorine | radon | nitrogen valence | 1 | 6 | 3 electronegativity | 3.98 | | 3.04 electron affinity | 328 kJ/mol (kilojoules per mole) | 0 kJ/mol (kilojoules per mole) | 7 kJ/mol (kilojoules per mole) first ionization energy | 1681 kJ/mol (kilojoules per mole) | 1037 kJ/mol (kilojoules per mole) | 1402.3 kJ/mol (kilojoules per mole) ionization energies | 1681 kJ/mol | 3374.2 kJ/mol | 6050.4 kJ/mol | 8407.7 kJ/mol | 11022.7 kJ/mol | 15164.1 kJ/mol | 17868 kJ/mol | 92038.1 kJ/mol | 106434.3 kJ/mol | 1037 kJ/mol | 1402.3 kJ/mol | 2856 kJ/mol | 4578.1 kJ/mol | 7475 kJ/mol | 9444.9 kJ/mol | 53266.6 kJ/mol | 64360 kJ/mol
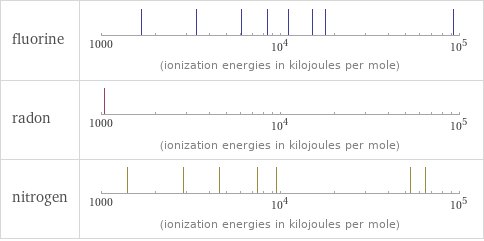
Reactivity
Atomic properties

| fluorine | radon | nitrogen term symbol | ^2P_(3/2) | ^1S_0 | ^4S_(3/2) atomic radius | 50 pm | 120 pm | 65 pm covalent radius | 57 pm | 150 pm | 71 pm van der Waals radius | 147 pm | | 155 pm (electronic ground state properties)
Abundances
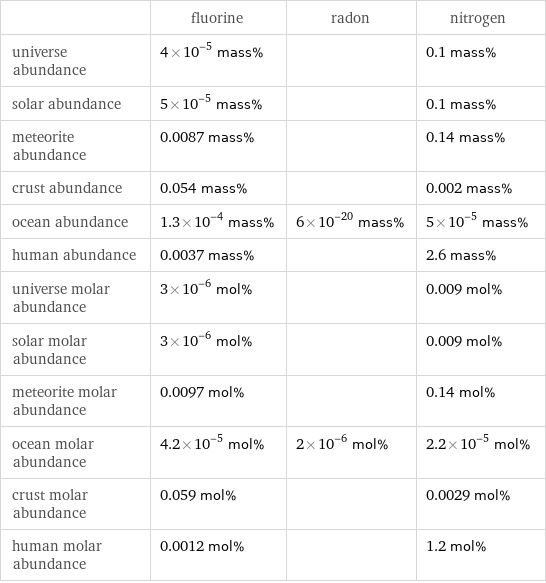
| fluorine | radon | nitrogen universe abundance | 4×10^-5 mass% | | 0.1 mass% solar abundance | 5×10^-5 mass% | | 0.1 mass% meteorite abundance | 0.0087 mass% | | 0.14 mass% crust abundance | 0.054 mass% | | 0.002 mass% ocean abundance | 1.3×10^-4 mass% | 6×10^-20 mass% | 5×10^-5 mass% human abundance | 0.0037 mass% | | 2.6 mass% universe molar abundance | 3×10^-6 mol% | | 0.009 mol% solar molar abundance | 3×10^-6 mol% | | 0.009 mol% meteorite molar abundance | 0.0097 mol% | | 0.14 mol% ocean molar abundance | 4.2×10^-5 mol% | 2×10^-6 mol% | 2.2×10^-5 mol% crust molar abundance | 0.059 mol% | | 0.0029 mol% human molar abundance | 0.0012 mol% | | 1.2 mol%