Input interpretation
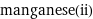
manganese(ii)
Basic properties
![molar mass | 54.94 g/mol formula | Mn^2+ empirical formula | Mn_ SMILES identifier | [Mn+2] InChI identifier | InChI=1/Mn/q+2 InChI key | WAEMQWOKJMHJLA-UHFFFAOYSA-N](../image_source/b2f206307edf88adf42bac8f9042ce6b.png)
molar mass | 54.94 g/mol formula | Mn^2+ empirical formula | Mn_ SMILES identifier | [Mn+2] InChI identifier | InChI=1/Mn/q+2 InChI key | WAEMQWOKJMHJLA-UHFFFAOYSA-N
Structure diagram
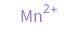
Structure diagram
Quantitative molecular descriptors
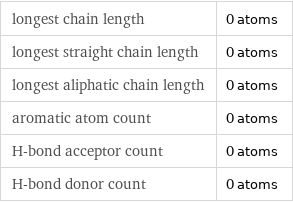
longest chain length | 0 atoms longest straight chain length | 0 atoms longest aliphatic chain length | 0 atoms aromatic atom count | 0 atoms H-bond acceptor count | 0 atoms H-bond donor count | 0 atoms
Elemental composition

| number of atoms | atom percent | mass percent Mn (manganese) | 1 | 100.0% | 100.00%
Elemental oxidation states
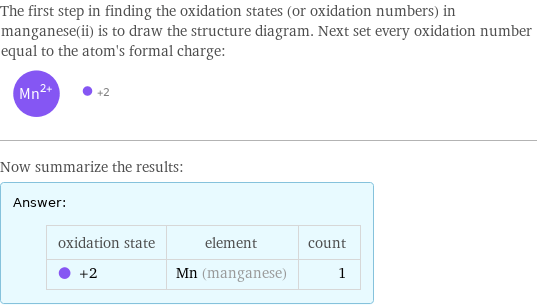
The first step in finding the oxidation states (or oxidation numbers) in manganese(ii) is to draw the structure diagram. Next set every oxidation number equal to the atom's formal charge: Now summarize the results: Answer: | | oxidation state | element | count +2 | Mn (manganese) | 1
Topological indices
vertex count | 1 edge count | 0 Schultz index | Wiener index | Hosoya index | Balaban index |