Input interpretation
scandium fluoride
Chemical names and formulas
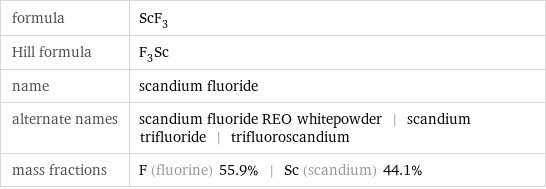
formula | ScF_3 Hill formula | F_3Sc name | scandium fluoride alternate names | scandium fluoride REO whitepowder | scandium trifluoride | trifluoroscandium mass fractions | F (fluorine) 55.9% | Sc (scandium) 44.1%
Structure diagram
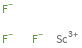
Structure diagram
Basic properties
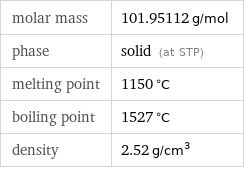
molar mass | 101.95112 g/mol phase | solid (at STP) melting point | 1150 °C boiling point | 1527 °C density | 2.52 g/cm^3
Units

Solid properties (at STP)
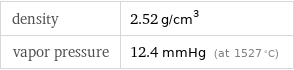
density | 2.52 g/cm^3 vapor pressure | 12.4 mmHg (at 1527 °C)
Units

Thermodynamic properties
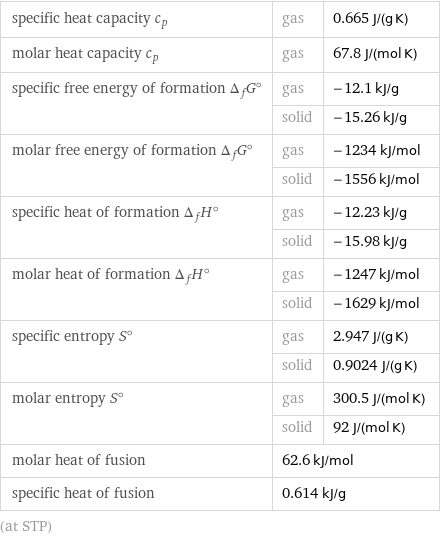
specific heat capacity c_p | gas | 0.665 J/(g K) molar heat capacity c_p | gas | 67.8 J/(mol K) specific free energy of formation Δ_fG° | gas | -12.1 kJ/g | solid | -15.26 kJ/g molar free energy of formation Δ_fG° | gas | -1234 kJ/mol | solid | -1556 kJ/mol specific heat of formation Δ_fH° | gas | -12.23 kJ/g | solid | -15.98 kJ/g molar heat of formation Δ_fH° | gas | -1247 kJ/mol | solid | -1629 kJ/mol specific entropy S° | gas | 2.947 J/(g K) | solid | 0.9024 J/(g K) molar entropy S° | gas | 300.5 J/(mol K) | solid | 92 J/(mol K) molar heat of fusion | 62.6 kJ/mol | specific heat of fusion | 0.614 kJ/g | (at STP)
Chemical identifiers
![CAS number | 13709-47-2 SMILES identifier | [F-].[F-].[F-].[Sc+3] RTECS number | VQ8930000 MDL number | MFCD00016325](../image_source/b590a382014d5da44e3db0b0880e0359.png)
CAS number | 13709-47-2 SMILES identifier | [F-].[F-].[F-].[Sc+3] RTECS number | VQ8930000 MDL number | MFCD00016325
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | other