Input interpretation
diamond (mineral)
Image

Image
General properties
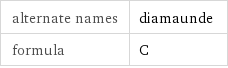
alternate names | diamaunde formula | C
Basic properties
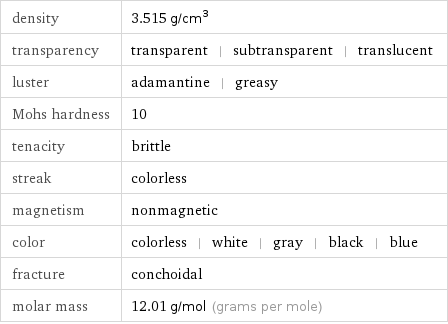
density | 3.515 g/cm^3 transparency | transparent | subtransparent | translucent luster | adamantine | greasy Mohs hardness | 10 tenacity | brittle streak | colorless magnetism | nonmagnetic color | colorless | white | gray | black | blue fracture | conchoidal molar mass | 12.01 g/mol (grams per mole)
Units

Mineral identifiers

Strunz ID | I/B.02-40 Dana ID | 1.3.6.1
Crystallographic properties
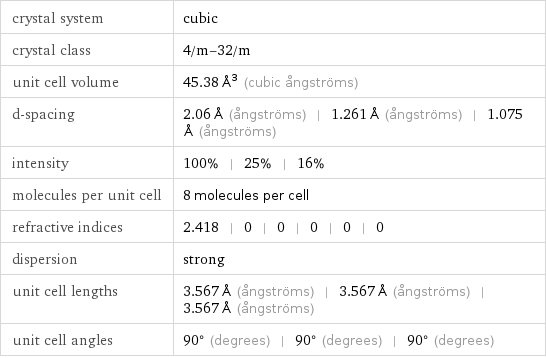
crystal system | cubic crystal class | 4/m-32/m unit cell volume | 45.38 Å^3 (cubic ångströms) d-spacing | 2.06 Å (ångströms) | 1.261 Å (ångströms) | 1.075 Å (ångströms) intensity | 100% | 25% | 16% molecules per unit cell | 8 molecules per cell refractive indices | 2.418 | 0 | 0 | 0 | 0 | 0 dispersion | strong unit cell lengths | 3.567 Å (ångströms) | 3.567 Å (ångströms) | 3.567 Å (ångströms) unit cell angles | 90° (degrees) | 90° (degrees) | 90° (degrees)
Wikipedia summary

Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. At room temperature and pressure, another solid form of carbon known as graphite is the chemically stable form, but diamond almost never converts to it. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are utilized in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth.