Input interpretation
zinc oxalate dihydrate | calcium chloride hydrate
Chemical names and formulas
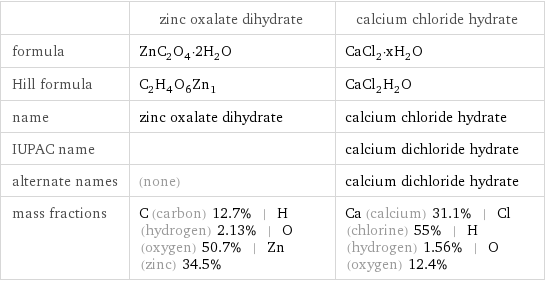
| zinc oxalate dihydrate | calcium chloride hydrate formula | ZnC_2O_4·2H_2O | CaCl_2·xH_2O Hill formula | C_2H_4O_6Zn_1 | CaCl_2H_2O name | zinc oxalate dihydrate | calcium chloride hydrate IUPAC name | | calcium dichloride hydrate alternate names | (none) | calcium dichloride hydrate mass fractions | C (carbon) 12.7% | H (hydrogen) 2.13% | O (oxygen) 50.7% | Zn (zinc) 34.5% | Ca (calcium) 31.1% | Cl (chlorine) 55% | H (hydrogen) 1.56% | O (oxygen) 12.4%
Structure diagrams
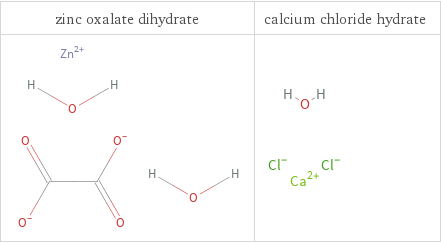
Structure diagrams
Basic properties
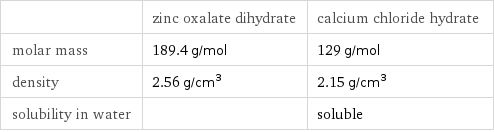
| zinc oxalate dihydrate | calcium chloride hydrate molar mass | 189.4 g/mol | 129 g/mol density | 2.56 g/cm^3 | 2.15 g/cm^3 solubility in water | | soluble
Units
