Input interpretation
aluminum
Chemical names and formulas
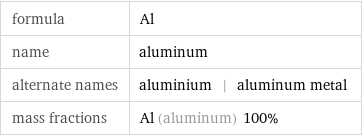
formula | Al name | aluminum alternate names | aluminium | aluminum metal mass fractions | Al (aluminum) 100%
Structure diagram

Structure diagram
Basic properties
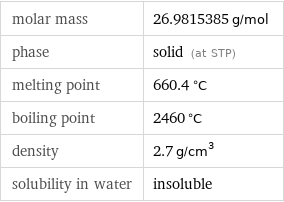
molar mass | 26.9815385 g/mol phase | solid (at STP) melting point | 660.4 °C boiling point | 2460 °C density | 2.7 g/cm^3 solubility in water | insoluble
Units

Basic drug properties
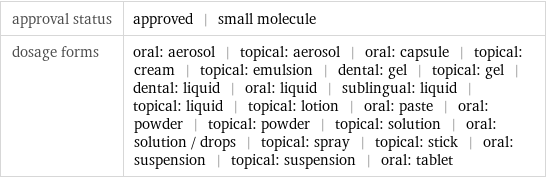
approval status | approved | small molecule dosage forms | oral: aerosol | topical: aerosol | oral: capsule | topical: cream | topical: emulsion | dental: gel | topical: gel | dental: liquid | oral: liquid | sublingual: liquid | topical: liquid | topical: lotion | oral: paste | oral: powder | topical: powder | topical: solution | oral: solution / drops | topical: spray | topical: stick | oral: suspension | topical: suspension | oral: tablet
Solid properties (at STP)

density | 2.7 g/cm^3 vapor pressure | 7×10^-29 mmHg (at 25 °C)
Units

Thermodynamic properties
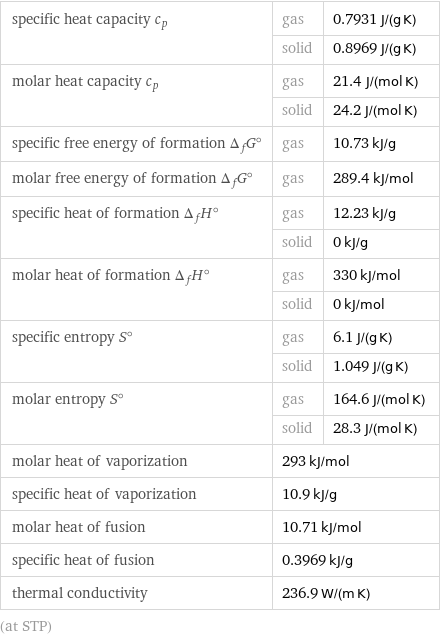
specific heat capacity c_p | gas | 0.7931 J/(g K) | solid | 0.8969 J/(g K) molar heat capacity c_p | gas | 21.4 J/(mol K) | solid | 24.2 J/(mol K) specific free energy of formation Δ_fG° | gas | 10.73 kJ/g molar free energy of formation Δ_fG° | gas | 289.4 kJ/mol specific heat of formation Δ_fH° | gas | 12.23 kJ/g | solid | 0 kJ/g molar heat of formation Δ_fH° | gas | 330 kJ/mol | solid | 0 kJ/mol specific entropy S° | gas | 6.1 J/(g K) | solid | 1.049 J/(g K) molar entropy S° | gas | 164.6 J/(mol K) | solid | 28.3 J/(mol K) molar heat of vaporization | 293 kJ/mol | specific heat of vaporization | 10.9 kJ/g | molar heat of fusion | 10.71 kJ/mol | specific heat of fusion | 0.3969 kJ/g | thermal conductivity | 236.9 W/(m K) | (at STP)
Chemical identifiers
![CAS number | 7429-90-5 PubChem CID number | 5359268 PubChem SID number | 24852100 SMILES identifier | [Al] InChI identifier | InChI=1/Al InChI key | XAGFODPZIPBFFR-UHFFFAOYAX RTECS number | BD0330000 MDL number | MFCD00134029](../image_source/d47b8ce99d1d24393263c93ba57a8d9a.png)
CAS number | 7429-90-5 PubChem CID number | 5359268 PubChem SID number | 24852100 SMILES identifier | [Al] InChI identifier | InChI=1/Al InChI key | XAGFODPZIPBFFR-UHFFFAOYAX RTECS number | BD0330000 MDL number | MFCD00134029
NFPA label

NFPA label

NFPA health rating | 0 NFPA fire rating | 1 NFPA reactivity rating | 1
Safety properties

flash point | 645 °C autoignition point | 400 °C

DOT hazard class | 4.3 DOT numbers | 1396
Toxicity properties

odor | odorless short-term exposure limit | 20 mg/m^3

long-term exposure limit | 10 mg/m^3 (over 8 hours) RTECS classes | other