Input interpretation
stable elements
Members

actinium | aluminum | antimony | argon | arsenic | barium | beryllium | bismuth | boron | bromine | cadmium | calcium | carbon | cerium | cesium | chlorine | chromium | cobalt | copper | dysprosium | erbium | europium | fluorine | gadolinium | gallium | germanium | gold | hafnium | helium | holmium | hydrogen | indium | iodine | iridium | iron | krypton | lanthanum | lead | lithium | lutetium | ... (total: 82)
Periodic table location
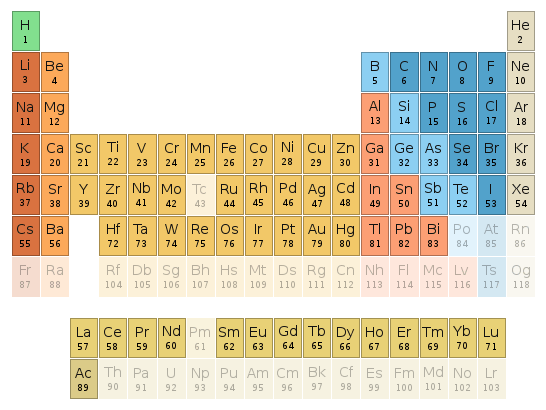
Periodic table location
Images
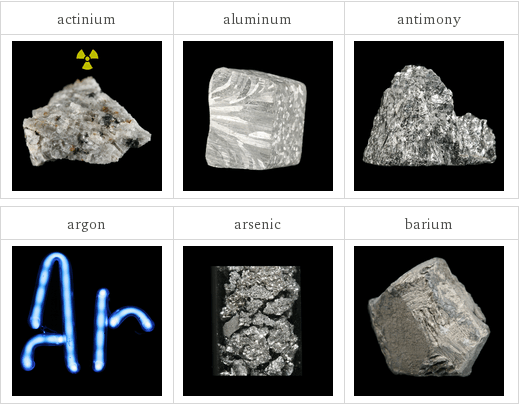
Images
Basic elemental properties
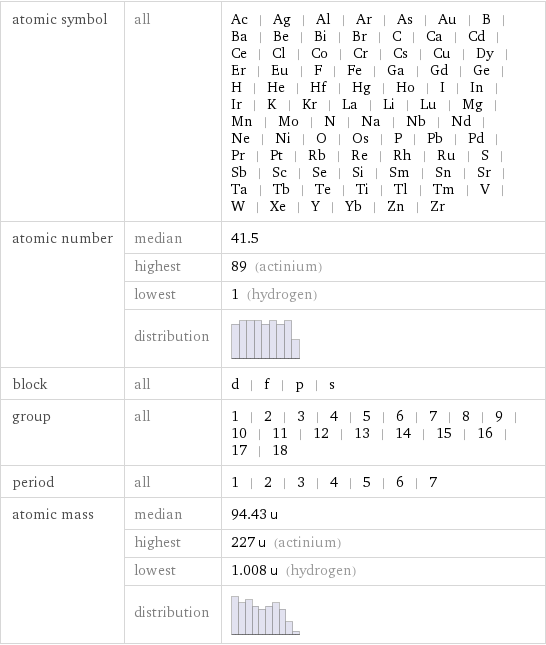
atomic symbol | all | Ac | Ag | Al | Ar | As | Au | B | Ba | Be | Bi | Br | C | Ca | Cd | Ce | Cl | Co | Cr | Cs | Cu | Dy | Er | Eu | F | Fe | Ga | Gd | Ge | H | He | Hf | Hg | Ho | I | In | Ir | K | Kr | La | Li | Lu | Mg | Mn | Mo | N | Na | Nb | Nd | Ne | Ni | O | Os | P | Pb | Pd | Pr | Pt | Rb | Re | Rh | Ru | S | Sb | Sc | Se | Si | Sm | Sn | Sr | Ta | Tb | Te | Ti | Tl | Tm | V | W | Xe | Y | Yb | Zn | Zr atomic number | median | 41.5 | highest | 89 (actinium) | lowest | 1 (hydrogen) | distribution | block | all | d | f | p | s group | all | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 period | all | 1 | 2 | 3 | 4 | 5 | 6 | 7 atomic mass | median | 94.43 u | highest | 227 u (actinium) | lowest | 1.008 u (hydrogen) | distribution |
Thermodynamic properties
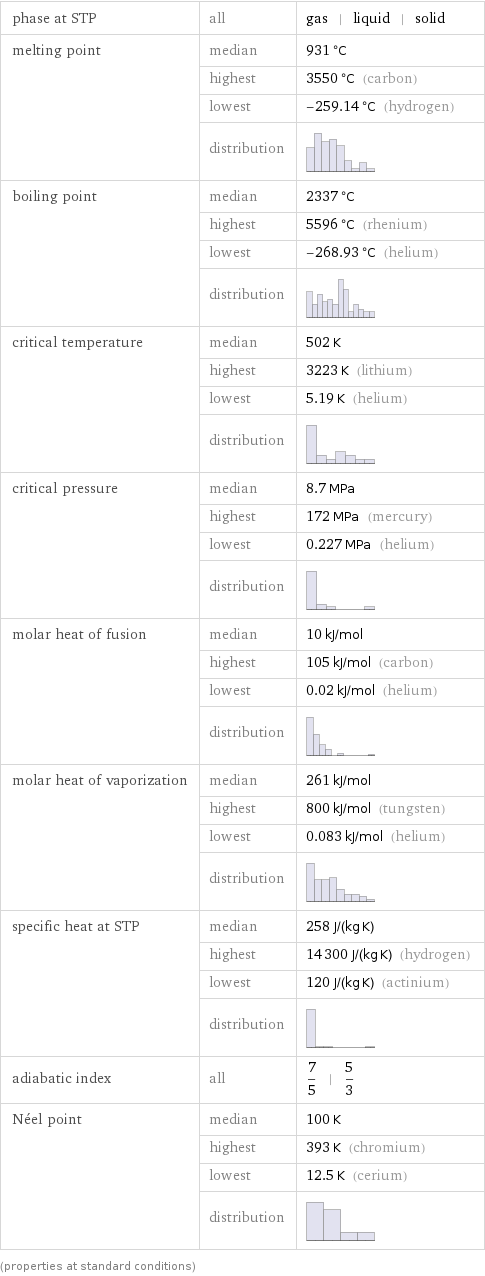
phase at STP | all | gas | liquid | solid melting point | median | 931 °C | highest | 3550 °C (carbon) | lowest | -259.14 °C (hydrogen) | distribution | boiling point | median | 2337 °C | highest | 5596 °C (rhenium) | lowest | -268.93 °C (helium) | distribution | critical temperature | median | 502 K | highest | 3223 K (lithium) | lowest | 5.19 K (helium) | distribution | critical pressure | median | 8.7 MPa | highest | 172 MPa (mercury) | lowest | 0.227 MPa (helium) | distribution | molar heat of fusion | median | 10 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.02 kJ/mol (helium) | distribution | molar heat of vaporization | median | 261 kJ/mol | highest | 800 kJ/mol (tungsten) | lowest | 0.083 kJ/mol (helium) | distribution | specific heat at STP | median | 258 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 120 J/(kg K) (actinium) | distribution | adiabatic index | all | 7/5 | 5/3 Néel point | median | 100 K | highest | 393 K (chromium) | lowest | 12.5 K (cerium) | distribution | (properties at standard conditions)
Material properties
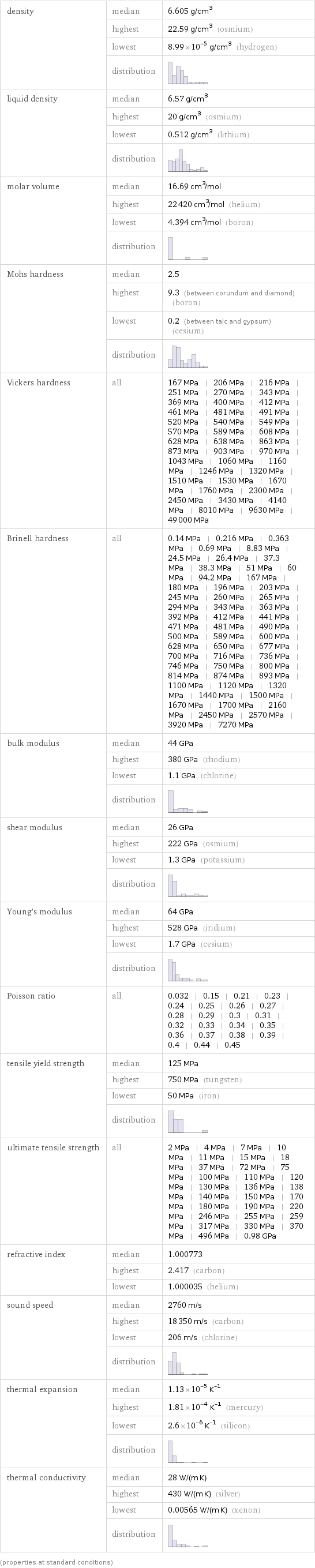
density | median | 6.605 g/cm^3 | highest | 22.59 g/cm^3 (osmium) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) | distribution | liquid density | median | 6.57 g/cm^3 | highest | 20 g/cm^3 (osmium) | lowest | 0.512 g/cm^3 (lithium) | distribution | molar volume | median | 16.69 cm^3/mol | highest | 22420 cm^3/mol (helium) | lowest | 4.394 cm^3/mol (boron) | distribution | Mohs hardness | median | 2.5 | highest | 9.3 (between corundum and diamond) (boron) | lowest | 0.2 (between talc and gypsum) (cesium) | distribution | Vickers hardness | all | 167 MPa | 206 MPa | 216 MPa | 251 MPa | 270 MPa | 343 MPa | 369 MPa | 400 MPa | 412 MPa | 461 MPa | 481 MPa | 491 MPa | 520 MPa | 540 MPa | 549 MPa | 570 MPa | 589 MPa | 608 MPa | 628 MPa | 638 MPa | 863 MPa | 873 MPa | 903 MPa | 970 MPa | 1043 MPa | 1060 MPa | 1160 MPa | 1246 MPa | 1320 MPa | 1510 MPa | 1530 MPa | 1670 MPa | 1760 MPa | 2300 MPa | 2450 MPa | 3430 MPa | 4140 MPa | 8010 MPa | 9630 MPa | 49000 MPa Brinell hardness | all | 0.14 MPa | 0.216 MPa | 0.363 MPa | 0.69 MPa | 8.83 MPa | 24.5 MPa | 26.4 MPa | 37.3 MPa | 38.3 MPa | 51 MPa | 60 MPa | 94.2 MPa | 167 MPa | 180 MPa | 196 MPa | 203 MPa | 245 MPa | 260 MPa | 265 MPa | 294 MPa | 343 MPa | 363 MPa | 392 MPa | 412 MPa | 441 MPa | 471 MPa | 481 MPa | 490 MPa | 500 MPa | 589 MPa | 600 MPa | 628 MPa | 650 MPa | 677 MPa | 700 MPa | 716 MPa | 736 MPa | 746 MPa | 750 MPa | 800 MPa | 814 MPa | 874 MPa | 893 MPa | 1100 MPa | 1120 MPa | 1320 MPa | 1440 MPa | 1500 MPa | 1670 MPa | 1700 MPa | 2160 MPa | 2450 MPa | 2570 MPa | 3920 MPa | 7270 MPa bulk modulus | median | 44 GPa | highest | 380 GPa (rhodium) | lowest | 1.1 GPa (chlorine) | distribution | shear modulus | median | 26 GPa | highest | 222 GPa (osmium) | lowest | 1.3 GPa (potassium) | distribution | Young's modulus | median | 64 GPa | highest | 528 GPa (iridium) | lowest | 1.7 GPa (cesium) | distribution | Poisson ratio | all | 0.032 | 0.15 | 0.21 | 0.23 | 0.24 | 0.25 | 0.26 | 0.27 | 0.28 | 0.29 | 0.3 | 0.31 | 0.32 | 0.33 | 0.34 | 0.35 | 0.36 | 0.37 | 0.38 | 0.39 | 0.4 | 0.44 | 0.45 tensile yield strength | median | 125 MPa | highest | 750 MPa (tungsten) | lowest | 50 MPa (iron) | distribution | ultimate tensile strength | all | 2 MPa | 4 MPa | 7 MPa | 10 MPa | 11 MPa | 15 MPa | 18 MPa | 37 MPa | 72 MPa | 75 MPa | 100 MPa | 110 MPa | 120 MPa | 130 MPa | 136 MPa | 138 MPa | 140 MPa | 150 MPa | 170 MPa | 180 MPa | 190 MPa | 220 MPa | 246 MPa | 255 MPa | 259 MPa | 317 MPa | 330 MPa | 370 MPa | 496 MPa | 0.98 GPa refractive index | median | 1.000773 | highest | 2.417 (carbon) | lowest | 1.000035 (helium) sound speed | median | 2760 m/s | highest | 18350 m/s (carbon) | lowest | 206 m/s (chlorine) | distribution | thermal expansion | median | 1.13×10^-5 K^(-1) | highest | 1.81×10^-4 K^(-1) (mercury) | lowest | 2.6×10^-6 K^(-1) (silicon) | distribution | thermal conductivity | median | 28 W/(m K) | highest | 430 W/(m K) (silver) | lowest | 0.00565 W/(m K) (xenon) | distribution | (properties at standard conditions)