Input interpretation
antimony (chemical element) | hydrogen (chemical element) | sulfur (chemical element) | bromine (chemical element)
Periodic table location
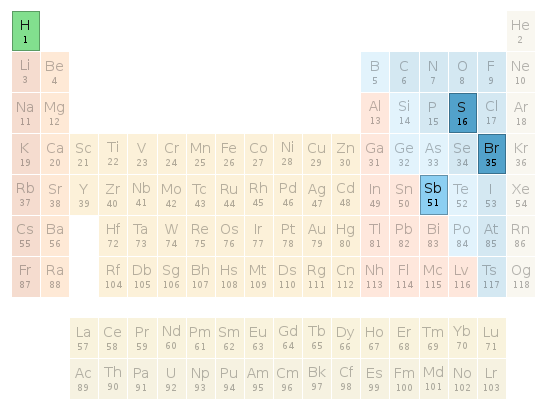
Periodic table location
Images
Images
Basic elemental properties
![| atomic symbol | atomic number | short electronic configuration antimony | Sb | 51 | [Kr]5s^24d^105p^3 hydrogen | H | 1 | 1s^1 sulfur | S | 16 | [Ne]3s^23p^4 bromine | Br | 35 | [Ar]4s^23d^104p^5 | Aufbau diagram | block | group antimony | 5p 4d 5s | p | 15 hydrogen | 1s | s | 1 sulfur | 3p 3s | p | 16 bromine | 4p 3d 4s | p | 17 | period | atomic mass | half-life antimony | 5 | 121.76 u | (stable) hydrogen | 1 | 1.008 u | (stable) sulfur | 3 | 32.06 u | (stable) bromine | 4 | 79.904 u | (stable)](../image_source/d7f3ebff8c7def2b7721bd0ac005e759.png)
| atomic symbol | atomic number | short electronic configuration antimony | Sb | 51 | [Kr]5s^24d^105p^3 hydrogen | H | 1 | 1s^1 sulfur | S | 16 | [Ne]3s^23p^4 bromine | Br | 35 | [Ar]4s^23d^104p^5 | Aufbau diagram | block | group antimony | 5p 4d 5s | p | 15 hydrogen | 1s | s | 1 sulfur | 3p 3s | p | 16 bromine | 4p 3d 4s | p | 17 | period | atomic mass | half-life antimony | 5 | 121.76 u | (stable) hydrogen | 1 | 1.008 u | (stable) sulfur | 3 | 32.06 u | (stable) bromine | 4 | 79.904 u | (stable)
Thermodynamic properties
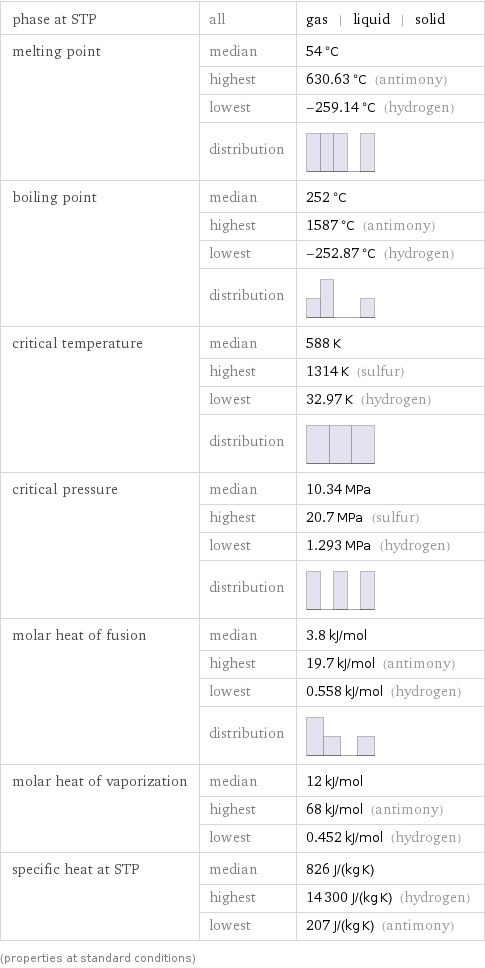
phase at STP | all | gas | liquid | solid melting point | median | 54 °C | highest | 630.63 °C (antimony) | lowest | -259.14 °C (hydrogen) | distribution | boiling point | median | 252 °C | highest | 1587 °C (antimony) | lowest | -252.87 °C (hydrogen) | distribution | critical temperature | median | 588 K | highest | 1314 K (sulfur) | lowest | 32.97 K (hydrogen) | distribution | critical pressure | median | 10.34 MPa | highest | 20.7 MPa (sulfur) | lowest | 1.293 MPa (hydrogen) | distribution | molar heat of fusion | median | 3.8 kJ/mol | highest | 19.7 kJ/mol (antimony) | lowest | 0.558 kJ/mol (hydrogen) | distribution | molar heat of vaporization | median | 12 kJ/mol | highest | 68 kJ/mol (antimony) | lowest | 0.452 kJ/mol (hydrogen) specific heat at STP | median | 826 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 207 J/(kg K) (antimony) (properties at standard conditions)
Material properties
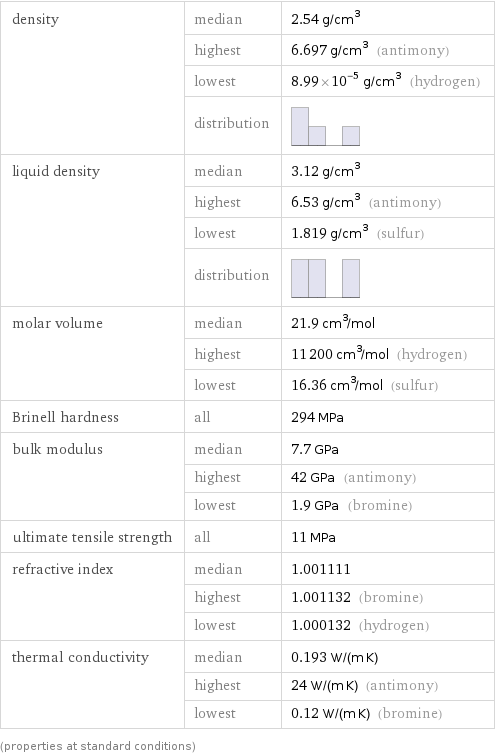
density | median | 2.54 g/cm^3 | highest | 6.697 g/cm^3 (antimony) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) | distribution | liquid density | median | 3.12 g/cm^3 | highest | 6.53 g/cm^3 (antimony) | lowest | 1.819 g/cm^3 (sulfur) | distribution | molar volume | median | 21.9 cm^3/mol | highest | 11200 cm^3/mol (hydrogen) | lowest | 16.36 cm^3/mol (sulfur) Brinell hardness | all | 294 MPa bulk modulus | median | 7.7 GPa | highest | 42 GPa (antimony) | lowest | 1.9 GPa (bromine) ultimate tensile strength | all | 11 MPa refractive index | median | 1.001111 | highest | 1.001132 (bromine) | lowest | 1.000132 (hydrogen) thermal conductivity | median | 0.193 W/(m K) | highest | 24 W/(m K) (antimony) | lowest | 0.12 W/(m K) (bromine) (properties at standard conditions)
Electromagnetic properties
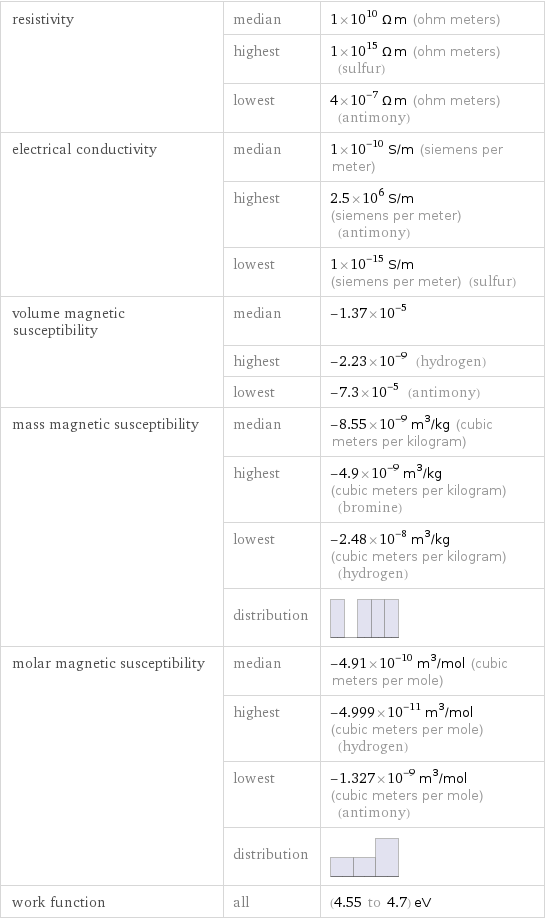
resistivity | median | 1×10^10 Ω m (ohm meters) | highest | 1×10^15 Ω m (ohm meters) (sulfur) | lowest | 4×10^-7 Ω m (ohm meters) (antimony) electrical conductivity | median | 1×10^-10 S/m (siemens per meter) | highest | 2.5×10^6 S/m (siemens per meter) (antimony) | lowest | 1×10^-15 S/m (siemens per meter) (sulfur) volume magnetic susceptibility | median | -1.37×10^-5 | highest | -2.23×10^-9 (hydrogen) | lowest | -7.3×10^-5 (antimony) mass magnetic susceptibility | median | -8.55×10^-9 m^3/kg (cubic meters per kilogram) | highest | -4.9×10^-9 m^3/kg (cubic meters per kilogram) (bromine) | lowest | -2.48×10^-8 m^3/kg (cubic meters per kilogram) (hydrogen) | distribution | molar magnetic susceptibility | median | -4.91×10^-10 m^3/mol (cubic meters per mole) | highest | -4.999×10^-11 m^3/mol (cubic meters per mole) (hydrogen) | lowest | -1.327×10^-9 m^3/mol (cubic meters per mole) (antimony) | distribution | work function | all | (4.55 to 4.7) eV
Reactivity
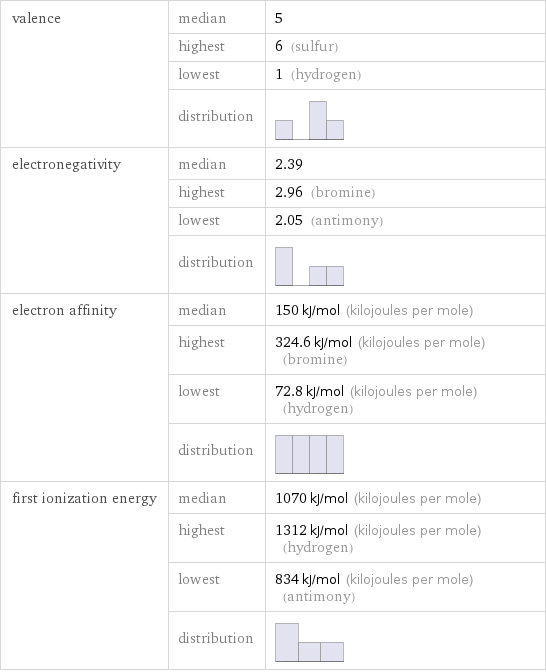
valence | median | 5 | highest | 6 (sulfur) | lowest | 1 (hydrogen) | distribution | electronegativity | median | 2.39 | highest | 2.96 (bromine) | lowest | 2.05 (antimony) | distribution | electron affinity | median | 150 kJ/mol (kilojoules per mole) | highest | 324.6 kJ/mol (kilojoules per mole) (bromine) | lowest | 72.8 kJ/mol (kilojoules per mole) (hydrogen) | distribution | first ionization energy | median | 1070 kJ/mol (kilojoules per mole) | highest | 1312 kJ/mol (kilojoules per mole) (hydrogen) | lowest | 834 kJ/mol (kilojoules per mole) (antimony) | distribution |
Atomic properties
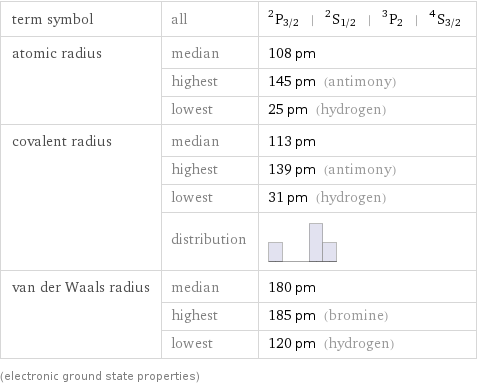
term symbol | all | ^2P_(3/2) | ^2S_(1/2) | ^3P_2 | ^4S_(3/2) atomic radius | median | 108 pm | highest | 145 pm (antimony) | lowest | 25 pm (hydrogen) covalent radius | median | 113 pm | highest | 139 pm (antimony) | lowest | 31 pm (hydrogen) | distribution | van der Waals radius | median | 180 pm | highest | 185 pm (bromine) | lowest | 120 pm (hydrogen) (electronic ground state properties)