Input interpretation
solid elements (at STP)
Members

lithium | beryllium | boron | carbon | sodium | magnesium | aluminum | silicon | phosphorus | sulfur | potassium | calcium | scandium | titanium | vanadium | chromium | manganese | iron | cobalt | nickel | copper | zinc | gallium | germanium | arsenic | selenium | rubidium | strontium | yttrium | zirconium | niobium | molybdenum | technetium | ruthenium | rhodium | palladium | silver | cadmium | indium | tin | ... (total: 86)
Periodic table location
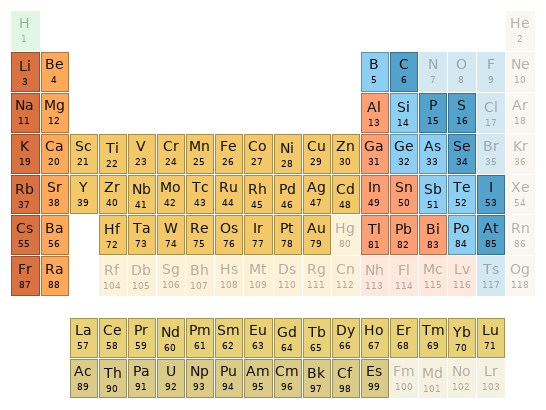
Periodic table location
Images

Images
Basic elemental properties
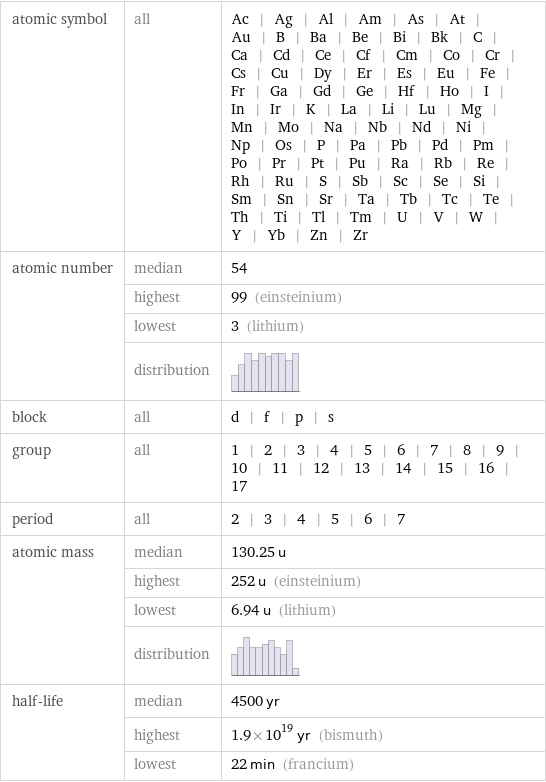
atomic symbol | all | Ac | Ag | Al | Am | As | At | Au | B | Ba | Be | Bi | Bk | C | Ca | Cd | Ce | Cf | Cm | Co | Cr | Cs | Cu | Dy | Er | Es | Eu | Fe | Fr | Ga | Gd | Ge | Hf | Ho | I | In | Ir | K | La | Li | Lu | Mg | Mn | Mo | Na | Nb | Nd | Ni | Np | Os | P | Pa | Pb | Pd | Pm | Po | Pr | Pt | Pu | Ra | Rb | Re | Rh | Ru | S | Sb | Sc | Se | Si | Sm | Sn | Sr | Ta | Tb | Tc | Te | Th | Ti | Tl | Tm | U | V | W | Y | Yb | Zn | Zr atomic number | median | 54 | highest | 99 (einsteinium) | lowest | 3 (lithium) | distribution | block | all | d | f | p | s group | all | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 period | all | 2 | 3 | 4 | 5 | 6 | 7 atomic mass | median | 130.25 u | highest | 252 u (einsteinium) | lowest | 6.94 u (lithium) | distribution | half-life | median | 4500 yr | highest | 1.9×10^19 yr (bismuth) | lowest | 22 min (francium)
Thermodynamic properties
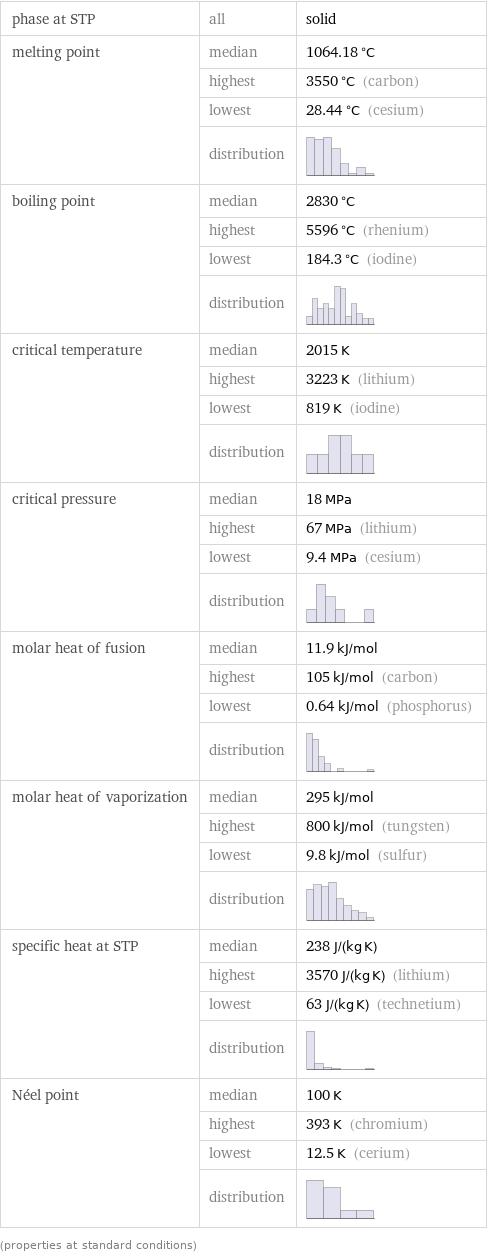
phase at STP | all | solid melting point | median | 1064.18 °C | highest | 3550 °C (carbon) | lowest | 28.44 °C (cesium) | distribution | boiling point | median | 2830 °C | highest | 5596 °C (rhenium) | lowest | 184.3 °C (iodine) | distribution | critical temperature | median | 2015 K | highest | 3223 K (lithium) | lowest | 819 K (iodine) | distribution | critical pressure | median | 18 MPa | highest | 67 MPa (lithium) | lowest | 9.4 MPa (cesium) | distribution | molar heat of fusion | median | 11.9 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.64 kJ/mol (phosphorus) | distribution | molar heat of vaporization | median | 295 kJ/mol | highest | 800 kJ/mol (tungsten) | lowest | 9.8 kJ/mol (sulfur) | distribution | specific heat at STP | median | 238 J/(kg K) | highest | 3570 J/(kg K) (lithium) | lowest | 63 J/(kg K) (technetium) | distribution | Néel point | median | 100 K | highest | 393 K (chromium) | lowest | 12.5 K (cerium) | distribution | (properties at standard conditions)
Material properties
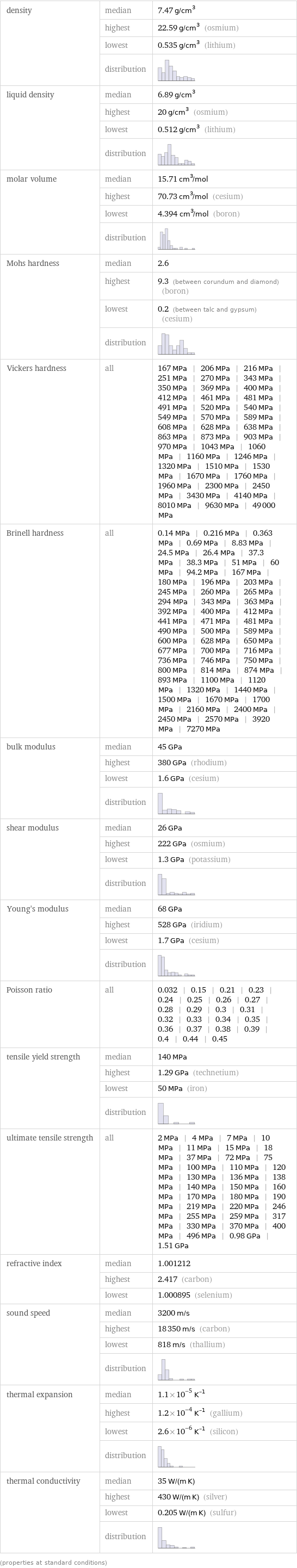
density | median | 7.47 g/cm^3 | highest | 22.59 g/cm^3 (osmium) | lowest | 0.535 g/cm^3 (lithium) | distribution | liquid density | median | 6.89 g/cm^3 | highest | 20 g/cm^3 (osmium) | lowest | 0.512 g/cm^3 (lithium) | distribution | molar volume | median | 15.71 cm^3/mol | highest | 70.73 cm^3/mol (cesium) | lowest | 4.394 cm^3/mol (boron) | distribution | Mohs hardness | median | 2.6 | highest | 9.3 (between corundum and diamond) (boron) | lowest | 0.2 (between talc and gypsum) (cesium) | distribution | Vickers hardness | all | 167 MPa | 206 MPa | 216 MPa | 251 MPa | 270 MPa | 343 MPa | 350 MPa | 369 MPa | 400 MPa | 412 MPa | 461 MPa | 481 MPa | 491 MPa | 520 MPa | 540 MPa | 549 MPa | 570 MPa | 589 MPa | 608 MPa | 628 MPa | 638 MPa | 863 MPa | 873 MPa | 903 MPa | 970 MPa | 1043 MPa | 1060 MPa | 1160 MPa | 1246 MPa | 1320 MPa | 1510 MPa | 1530 MPa | 1670 MPa | 1760 MPa | 1960 MPa | 2300 MPa | 2450 MPa | 3430 MPa | 4140 MPa | 8010 MPa | 9630 MPa | 49000 MPa Brinell hardness | all | 0.14 MPa | 0.216 MPa | 0.363 MPa | 0.69 MPa | 8.83 MPa | 24.5 MPa | 26.4 MPa | 37.3 MPa | 38.3 MPa | 51 MPa | 60 MPa | 94.2 MPa | 167 MPa | 180 MPa | 196 MPa | 203 MPa | 245 MPa | 260 MPa | 265 MPa | 294 MPa | 343 MPa | 363 MPa | 392 MPa | 400 MPa | 412 MPa | 441 MPa | 471 MPa | 481 MPa | 490 MPa | 500 MPa | 589 MPa | 600 MPa | 628 MPa | 650 MPa | 677 MPa | 700 MPa | 716 MPa | 736 MPa | 746 MPa | 750 MPa | 800 MPa | 814 MPa | 874 MPa | 893 MPa | 1100 MPa | 1120 MPa | 1320 MPa | 1440 MPa | 1500 MPa | 1670 MPa | 1700 MPa | 2160 MPa | 2400 MPa | 2450 MPa | 2570 MPa | 3920 MPa | 7270 MPa bulk modulus | median | 45 GPa | highest | 380 GPa (rhodium) | lowest | 1.6 GPa (cesium) | distribution | shear modulus | median | 26 GPa | highest | 222 GPa (osmium) | lowest | 1.3 GPa (potassium) | distribution | Young's modulus | median | 68 GPa | highest | 528 GPa (iridium) | lowest | 1.7 GPa (cesium) | distribution | Poisson ratio | all | 0.032 | 0.15 | 0.21 | 0.23 | 0.24 | 0.25 | 0.26 | 0.27 | 0.28 | 0.29 | 0.3 | 0.31 | 0.32 | 0.33 | 0.34 | 0.35 | 0.36 | 0.37 | 0.38 | 0.39 | 0.4 | 0.44 | 0.45 tensile yield strength | median | 140 MPa | highest | 1.29 GPa (technetium) | lowest | 50 MPa (iron) | distribution | ultimate tensile strength | all | 2 MPa | 4 MPa | 7 MPa | 10 MPa | 11 MPa | 15 MPa | 18 MPa | 37 MPa | 72 MPa | 75 MPa | 100 MPa | 110 MPa | 120 MPa | 130 MPa | 136 MPa | 138 MPa | 140 MPa | 150 MPa | 160 MPa | 170 MPa | 180 MPa | 190 MPa | 219 MPa | 220 MPa | 246 MPa | 255 MPa | 259 MPa | 317 MPa | 330 MPa | 370 MPa | 400 MPa | 496 MPa | 0.98 GPa | 1.51 GPa refractive index | median | 1.001212 | highest | 2.417 (carbon) | lowest | 1.000895 (selenium) sound speed | median | 3200 m/s | highest | 18350 m/s (carbon) | lowest | 818 m/s (thallium) | distribution | thermal expansion | median | 1.1×10^-5 K^(-1) | highest | 1.2×10^-4 K^(-1) (gallium) | lowest | 2.6×10^-6 K^(-1) (silicon) | distribution | thermal conductivity | median | 35 W/(m K) | highest | 430 W/(m K) (silver) | lowest | 0.205 W/(m K) (sulfur) | distribution | (properties at standard conditions)