Input interpretation
potassium iodide | sodium bromate
Chemical names and formulas
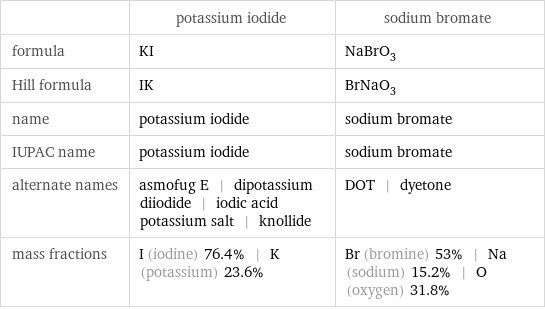
| potassium iodide | sodium bromate formula | KI | NaBrO_3 Hill formula | IK | BrNaO_3 name | potassium iodide | sodium bromate IUPAC name | potassium iodide | sodium bromate alternate names | asmofug E | dipotassium diiodide | iodic acid potassium salt | knollide | DOT | dyetone mass fractions | I (iodine) 76.4% | K (potassium) 23.6% | Br (bromine) 53% | Na (sodium) 15.2% | O (oxygen) 31.8%
Structure diagrams
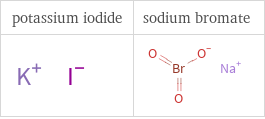
Structure diagrams
Basic properties
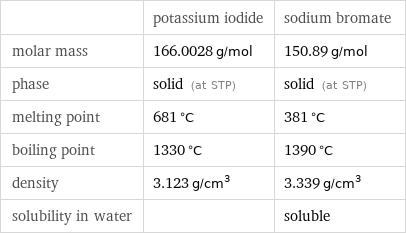
| potassium iodide | sodium bromate molar mass | 166.0028 g/mol | 150.89 g/mol phase | solid (at STP) | solid (at STP) melting point | 681 °C | 381 °C boiling point | 1330 °C | 1390 °C density | 3.123 g/cm^3 | 3.339 g/cm^3 solubility in water | | soluble
Units

Thermodynamic properties
| potassium iodide | sodium bromate molar heat of fusion | 24 kJ/mol (kilojoules per mole) | 28.11 kJ/mol (kilojoules per mole) specific heat of fusion | 0.14 kJ/g (kilojoules per gram) | 0.1863 kJ/g (kilojoules per gram)
Solid properties (at STP)

| potassium iodide | sodium bromate density | 3.123 g/cm^3 | 3.339 g/cm^3 vapor pressure | 0.9998 mmHg |
Units

Chemical identifiers
![| potassium iodide | sodium bromate CAS number | 7681-11-0 | 7789-38-0 PubChem CID number | 4875 | 23668195 PubChem SID number | 24852091 | 24853461 SMILES identifier | [K+].[I-] | [O-]Br(=O)=O.[Na+]](../image_source/c12e0db6715ebaf3c388fb39bad84fe1.png)
| potassium iodide | sodium bromate CAS number | 7681-11-0 | 7789-38-0 PubChem CID number | 4875 | 23668195 PubChem SID number | 24852091 | 24853461 SMILES identifier | [K+].[I-] | [O-]Br(=O)=O.[Na+]
NFPA label
NFPA label