Input interpretation
arsenic (chemical element) | nickel (chemical element) | copper (chemical element)
Periodic table location
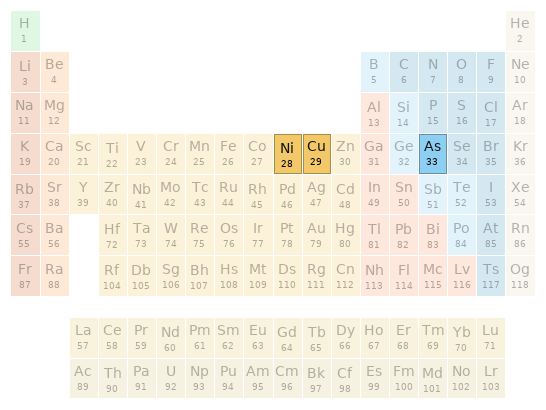
Periodic table location
Images

Images
Basic elemental properties
![| arsenic | nickel | copper atomic symbol | As | Ni | Cu atomic number | 33 | 28 | 29 short electronic configuration | [Ar]4s^23d^104p^3 | [Ar]4s^23d^8 | [Ar]4s^13d^10 Aufbau diagram | 4p 3d 4s | 3d 4s | 3d 4s block | p | d | d group | 15 | 10 | 11 period | 4 | 4 | 4 atomic mass | 74.921595 u | 58.6934 u | 63.546 u half-life | (stable) | (stable) | (stable)](../image_source/968cb3183448321d4c9ce528c4e7f6cd.png)
| arsenic | nickel | copper atomic symbol | As | Ni | Cu atomic number | 33 | 28 | 29 short electronic configuration | [Ar]4s^23d^104p^3 | [Ar]4s^23d^8 | [Ar]4s^13d^10 Aufbau diagram | 4p 3d 4s | 3d 4s | 3d 4s block | p | d | d group | 15 | 10 | 11 period | 4 | 4 | 4 atomic mass | 74.921595 u | 58.6934 u | 63.546 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
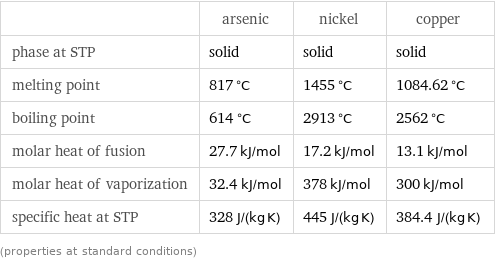
| arsenic | nickel | copper phase at STP | solid | solid | solid melting point | 817 °C | 1455 °C | 1084.62 °C boiling point | 614 °C | 2913 °C | 2562 °C molar heat of fusion | 27.7 kJ/mol | 17.2 kJ/mol | 13.1 kJ/mol molar heat of vaporization | 32.4 kJ/mol | 378 kJ/mol | 300 kJ/mol specific heat at STP | 328 J/(kg K) | 445 J/(kg K) | 384.4 J/(kg K) (properties at standard conditions)