Input interpretation

Lewis acids | mass fractions
Table
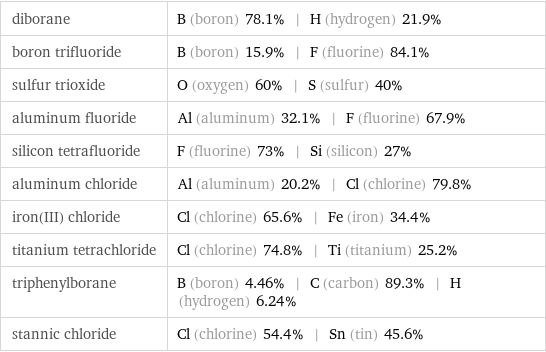
diborane | B (boron) 78.1% | H (hydrogen) 21.9% boron trifluoride | B (boron) 15.9% | F (fluorine) 84.1% sulfur trioxide | O (oxygen) 60% | S (sulfur) 40% aluminum fluoride | Al (aluminum) 32.1% | F (fluorine) 67.9% silicon tetrafluoride | F (fluorine) 73% | Si (silicon) 27% aluminum chloride | Al (aluminum) 20.2% | Cl (chlorine) 79.8% iron(III) chloride | Cl (chlorine) 65.6% | Fe (iron) 34.4% titanium tetrachloride | Cl (chlorine) 74.8% | Ti (titanium) 25.2% triphenylborane | B (boron) 4.46% | C (carbon) 89.3% | H (hydrogen) 6.24% stannic chloride | Cl (chlorine) 54.4% | Sn (tin) 45.6%