Input interpretation

Lewis acids | molecular mass
Summary
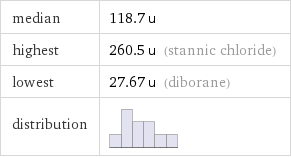
median | 118.7 u highest | 260.5 u (stannic chloride) lowest | 27.67 u (diborane) distribution |
Units

Distribution plots
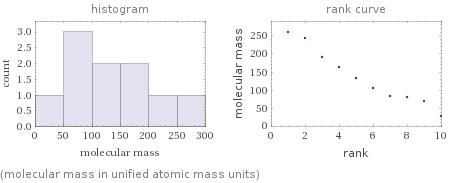
(molecular mass in unified atomic mass units)
Molecular mass rankings
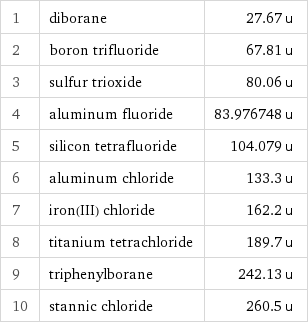
1 | diborane | 27.67 u 2 | boron trifluoride | 67.81 u 3 | sulfur trioxide | 80.06 u 4 | aluminum fluoride | 83.976748 u 5 | silicon tetrafluoride | 104.079 u 6 | aluminum chloride | 133.3 u 7 | iron(III) chloride | 162.2 u 8 | titanium tetrachloride | 189.7 u 9 | triphenylborane | 242.13 u 10 | stannic chloride | 260.5 u
Unit conversions for median molecular mass 118.7 u
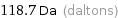
118.7 Da (daltons)
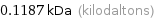
0.1187 kDa (kilodaltons)
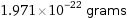
1.971×10^-22 grams
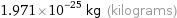
1.971×10^-25 kg (kilograms)

118.7 chemical atomic mass units (unit officially deprecated)

118.7 physical atomic mass units (unit officially deprecated)
Comparisons for median molecular mass 118.7 u
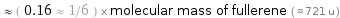
≈ ( 0.16 ≈ 1/6 ) × molecular mass of fullerene ( ≈ 721 u )
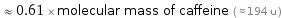
≈ 0.61 × molecular mass of caffeine ( ≈ 194 u )
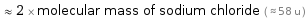
≈ 2 × molecular mass of sodium chloride ( ≈ 58 u )
Corresponding quantities
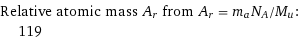
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 119
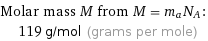
Molar mass M from M = m_aN_A: | 119 g/mol (grams per mole)
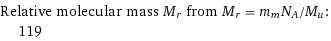
Relative molecular mass M_r from M_r = m_mN_A/M_u: | 119
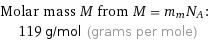
Molar mass M from M = m_mN_A: | 119 g/mol (grams per mole)