Input interpretation
calcium hydride
Chemical names and formulas

formula | CaH_2 name | calcium hydride alternate names | calcium dihydride mass fractions | Ca (calcium) 95.2% | H (hydrogen) 4.79%
Structure diagram
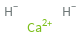
Structure diagram
Basic properties
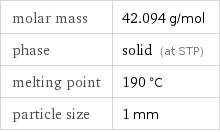
molar mass | 42.094 g/mol phase | solid (at STP) melting point | 190 °C particle size | 1 mm
Thermodynamic properties
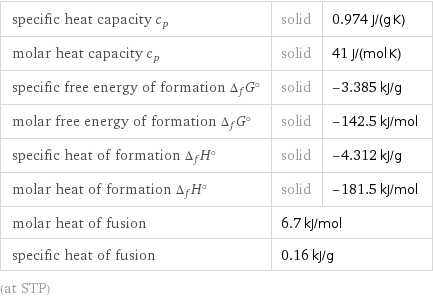
specific heat capacity c_p | solid | 0.974 J/(g K) molar heat capacity c_p | solid | 41 J/(mol K) specific free energy of formation Δ_fG° | solid | -3.385 kJ/g molar free energy of formation Δ_fG° | solid | -142.5 kJ/mol specific heat of formation Δ_fH° | solid | -4.312 kJ/g molar heat of formation Δ_fH° | solid | -181.5 kJ/mol molar heat of fusion | 6.7 kJ/mol | specific heat of fusion | 0.16 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7789-78-8 PubChem CID number | 105052 SMILES identifier | [H-].[H-].[Ca+2] InChI identifier | InChI=1/Ca.2H/q+2;2*-1 MDL number | MFCD00010897](../image_source/a3415bec764c2d803bb527f9b17f912f.png)
CAS number | 7789-78-8 PubChem CID number | 105052 SMILES identifier | [H-].[H-].[Ca+2] InChI identifier | InChI=1/Ca.2H/q+2;2*-1 MDL number | MFCD00010897
Ion equivalents

Ca^(2+) (calcium cation) | 1 H^+ (hydrogen cation) | 2