Input interpretation
1, 1, 1, 3, 3, 3-hexafluoropropane
Chemical names and formulas
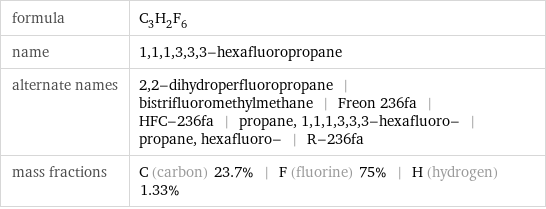
formula | C_3H_2F_6 name | 1, 1, 1, 3, 3, 3-hexafluoropropane alternate names | 2, 2-dihydroperfluoropropane | bistrifluoromethylmethane | Freon 236fa | HFC-236fa | propane, 1, 1, 1, 3, 3, 3-hexafluoro- | propane, hexafluoro- | R-236fa mass fractions | C (carbon) 23.7% | F (fluorine) 75% | H (hydrogen) 1.33%
Lewis structure
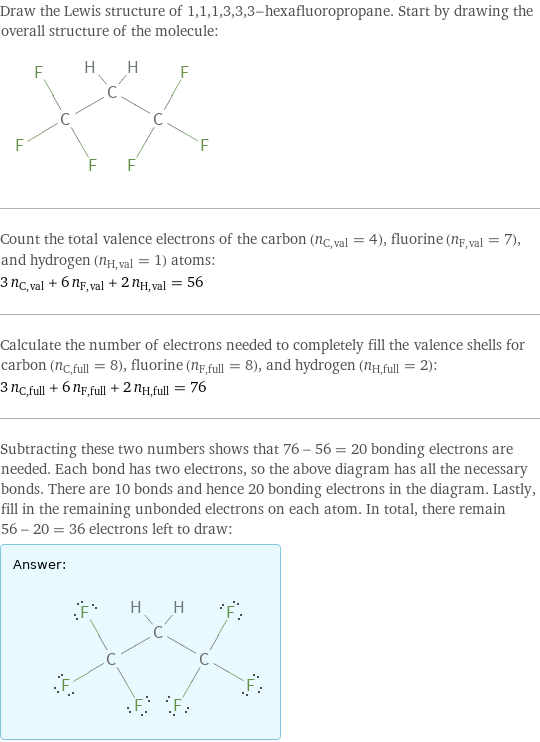
Draw the Lewis structure of 1, 1, 1, 3, 3, 3-hexafluoropropane. Start by drawing the overall structure of the molecule: Count the total valence electrons of the carbon (n_C, val = 4), fluorine (n_F, val = 7), and hydrogen (n_H, val = 1) atoms: 3 n_C, val + 6 n_F, val + 2 n_H, val = 56 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), fluorine (n_F, full = 8), and hydrogen (n_H, full = 2): 3 n_C, full + 6 n_F, full + 2 n_H, full = 76 Subtracting these two numbers shows that 76 - 56 = 20 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 10 bonds and hence 20 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 56 - 20 = 36 electrons left to draw: Answer: | |
3D structure
3D structure
Basic properties

molar mass | 152.04 g/mol phase | gas (at STP) boiling point | 0.8 °C density | 1.371 g/cm^3 (at 20 °C)
Units

Gas properties (at STP)
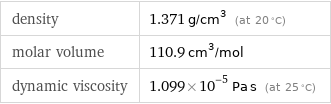
density | 1.371 g/cm^3 (at 20 °C) molar volume | 110.9 cm^3/mol dynamic viscosity | 1.099×10^-5 Pa s (at 25 °C)
Units
Thermodynamic properties

critical temperature | 398.1 K (at STP)
Phase diagram
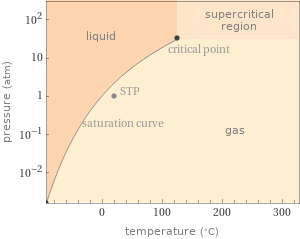
Phase diagram
Units

Chemical identifiers
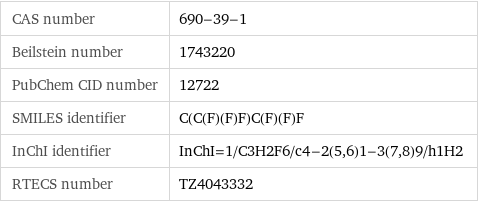
CAS number | 690-39-1 Beilstein number | 1743220 PubChem CID number | 12722 SMILES identifier | C(C(F)(F)F)C(F)(F)F InChI identifier | InChI=1/C3H2F6/c4-2(5, 6)1-3(7, 8)9/h1H2 RTECS number | TZ4043332
Toxicity properties

RTECS classes | other