Input interpretation

Lewis acids | element types
Summary

aluminum | boron | carbon | chlorine | fluorine | iron | hydrogen | oxygen | sulfur | silicon | tin | titanium
Periodic table location
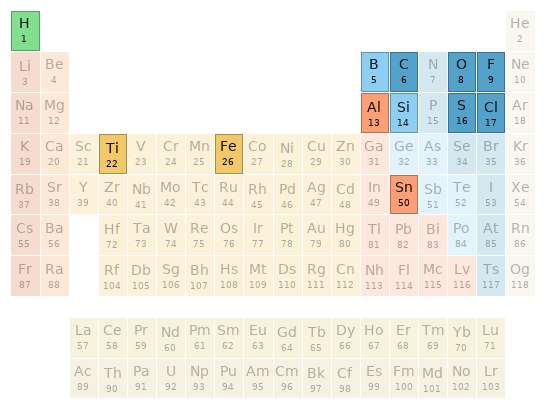
Periodic table location
Images

Images
Basic elemental properties
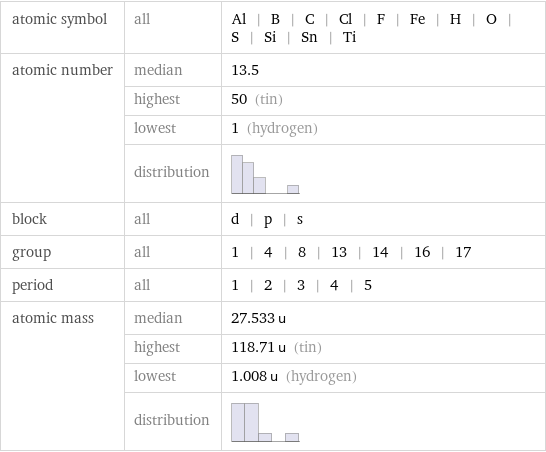
atomic symbol | all | Al | B | C | Cl | F | Fe | H | O | S | Si | Sn | Ti atomic number | median | 13.5 | highest | 50 (tin) | lowest | 1 (hydrogen) | distribution | block | all | d | p | s group | all | 1 | 4 | 8 | 13 | 14 | 16 | 17 period | all | 1 | 2 | 3 | 4 | 5 atomic mass | median | 27.533 u | highest | 118.71 u (tin) | lowest | 1.008 u (hydrogen) | distribution |
Table
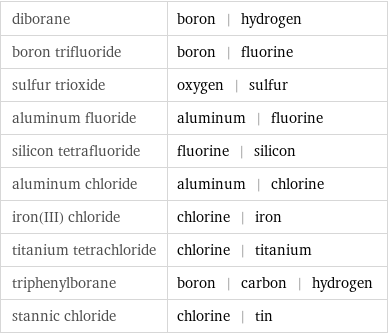
diborane | boron | hydrogen boron trifluoride | boron | fluorine sulfur trioxide | oxygen | sulfur aluminum fluoride | aluminum | fluorine silicon tetrafluoride | fluorine | silicon aluminum chloride | aluminum | chlorine iron(III) chloride | chlorine | iron titanium tetrachloride | chlorine | titanium triphenylborane | boron | carbon | hydrogen stannic chloride | chlorine | tin
Thermodynamic properties
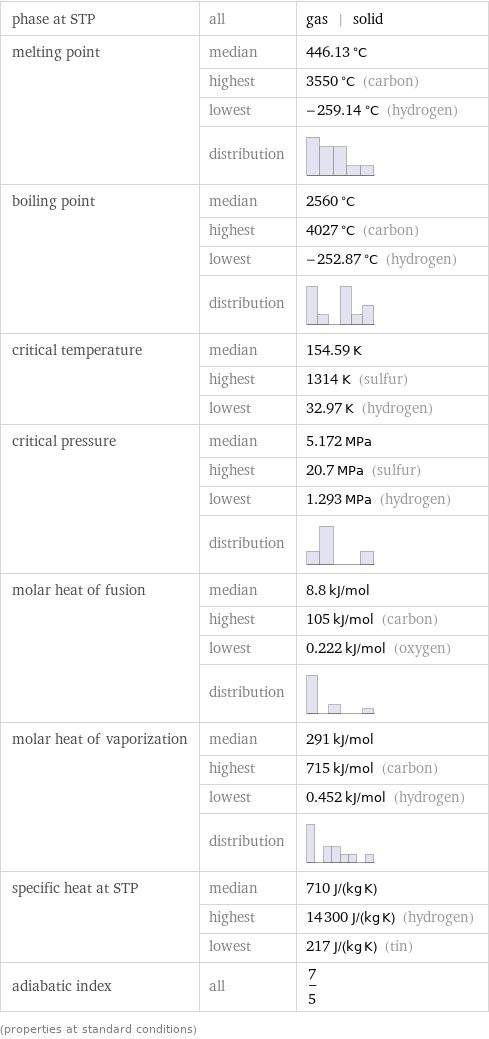
phase at STP | all | gas | solid melting point | median | 446.13 °C | highest | 3550 °C (carbon) | lowest | -259.14 °C (hydrogen) | distribution | boiling point | median | 2560 °C | highest | 4027 °C (carbon) | lowest | -252.87 °C (hydrogen) | distribution | critical temperature | median | 154.59 K | highest | 1314 K (sulfur) | lowest | 32.97 K (hydrogen) critical pressure | median | 5.172 MPa | highest | 20.7 MPa (sulfur) | lowest | 1.293 MPa (hydrogen) | distribution | molar heat of fusion | median | 8.8 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.222 kJ/mol (oxygen) | distribution | molar heat of vaporization | median | 291 kJ/mol | highest | 715 kJ/mol (carbon) | lowest | 0.452 kJ/mol (hydrogen) | distribution | specific heat at STP | median | 710 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 217 J/(kg K) (tin) adiabatic index | all | 7/5 (properties at standard conditions)
Material properties
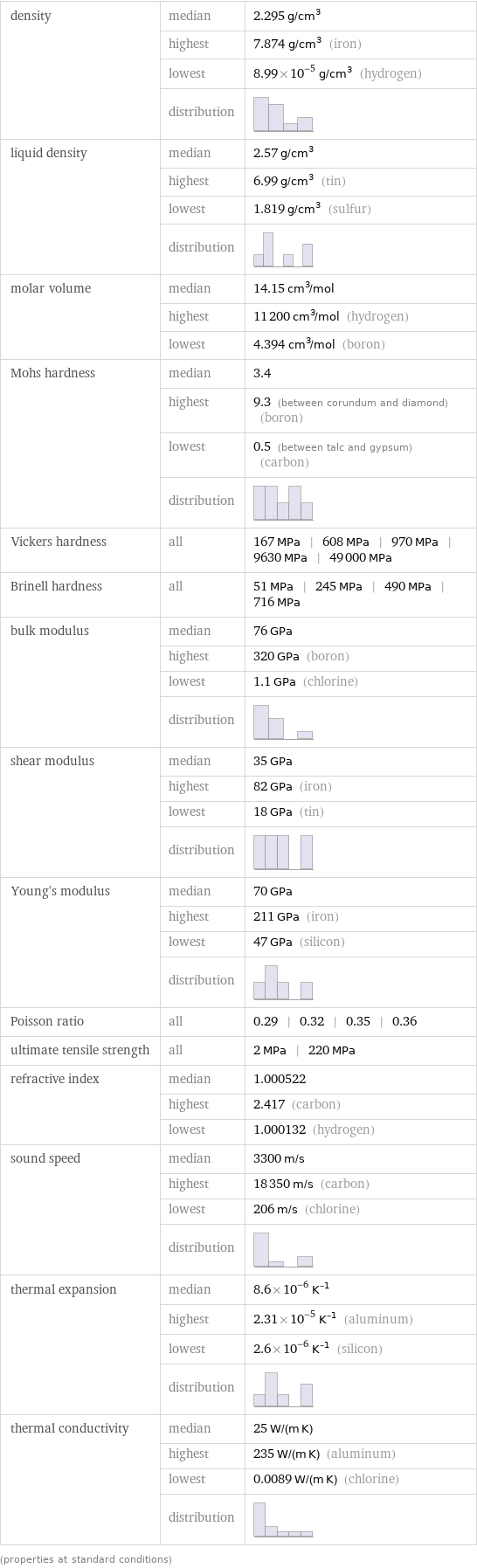
density | median | 2.295 g/cm^3 | highest | 7.874 g/cm^3 (iron) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) | distribution | liquid density | median | 2.57 g/cm^3 | highest | 6.99 g/cm^3 (tin) | lowest | 1.819 g/cm^3 (sulfur) | distribution | molar volume | median | 14.15 cm^3/mol | highest | 11200 cm^3/mol (hydrogen) | lowest | 4.394 cm^3/mol (boron) Mohs hardness | median | 3.4 | highest | 9.3 (between corundum and diamond) (boron) | lowest | 0.5 (between talc and gypsum) (carbon) | distribution | Vickers hardness | all | 167 MPa | 608 MPa | 970 MPa | 9630 MPa | 49000 MPa Brinell hardness | all | 51 MPa | 245 MPa | 490 MPa | 716 MPa bulk modulus | median | 76 GPa | highest | 320 GPa (boron) | lowest | 1.1 GPa (chlorine) | distribution | shear modulus | median | 35 GPa | highest | 82 GPa (iron) | lowest | 18 GPa (tin) | distribution | Young's modulus | median | 70 GPa | highest | 211 GPa (iron) | lowest | 47 GPa (silicon) | distribution | Poisson ratio | all | 0.29 | 0.32 | 0.35 | 0.36 ultimate tensile strength | all | 2 MPa | 220 MPa refractive index | median | 1.000522 | highest | 2.417 (carbon) | lowest | 1.000132 (hydrogen) sound speed | median | 3300 m/s | highest | 18350 m/s (carbon) | lowest | 206 m/s (chlorine) | distribution | thermal expansion | median | 8.6×10^-6 K^(-1) | highest | 2.31×10^-5 K^(-1) (aluminum) | lowest | 2.6×10^-6 K^(-1) (silicon) | distribution | thermal conductivity | median | 25 W/(m K) | highest | 235 W/(m K) (aluminum) | lowest | 0.0089 W/(m K) (chlorine) | distribution | (properties at standard conditions)
Electromagnetic properties
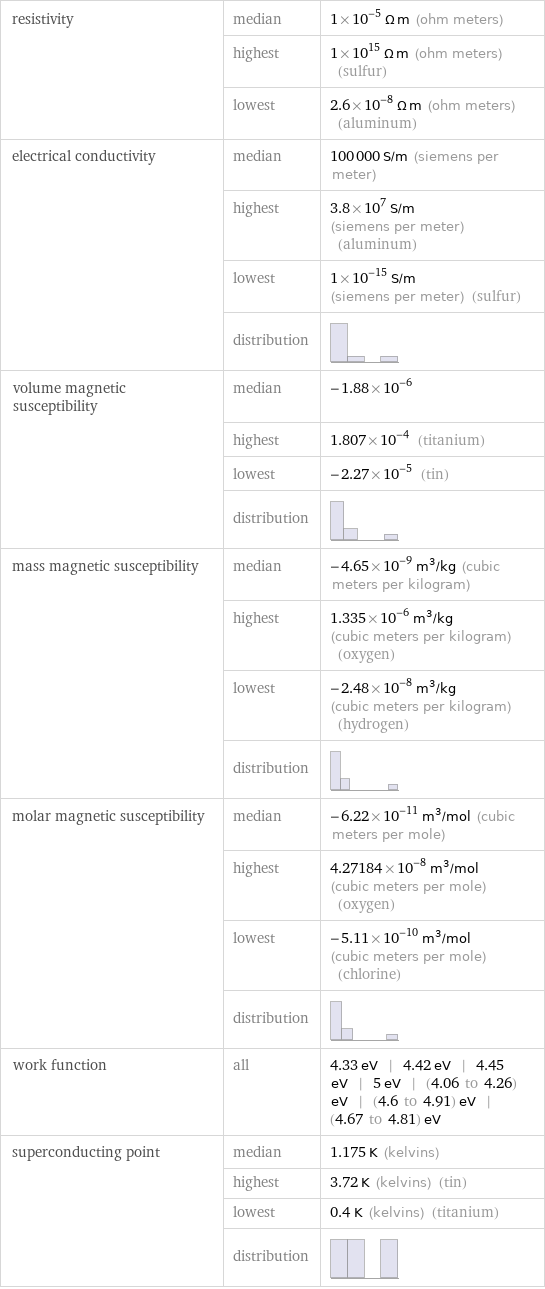
resistivity | median | 1×10^-5 Ω m (ohm meters) | highest | 1×10^15 Ω m (ohm meters) (sulfur) | lowest | 2.6×10^-8 Ω m (ohm meters) (aluminum) electrical conductivity | median | 100000 S/m (siemens per meter) | highest | 3.8×10^7 S/m (siemens per meter) (aluminum) | lowest | 1×10^-15 S/m (siemens per meter) (sulfur) | distribution | volume magnetic susceptibility | median | -1.88×10^-6 | highest | 1.807×10^-4 (titanium) | lowest | -2.27×10^-5 (tin) | distribution | mass magnetic susceptibility | median | -4.65×10^-9 m^3/kg (cubic meters per kilogram) | highest | 1.335×10^-6 m^3/kg (cubic meters per kilogram) (oxygen) | lowest | -2.48×10^-8 m^3/kg (cubic meters per kilogram) (hydrogen) | distribution | molar magnetic susceptibility | median | -6.22×10^-11 m^3/mol (cubic meters per mole) | highest | 4.27184×10^-8 m^3/mol (cubic meters per mole) (oxygen) | lowest | -5.11×10^-10 m^3/mol (cubic meters per mole) (chlorine) | distribution | work function | all | 4.33 eV | 4.42 eV | 4.45 eV | 5 eV | (4.06 to 4.26) eV | (4.6 to 4.91) eV | (4.67 to 4.81) eV superconducting point | median | 1.175 K (kelvins) | highest | 3.72 K (kelvins) (tin) | lowest | 0.4 K (kelvins) (titanium) | distribution |
Reactivity
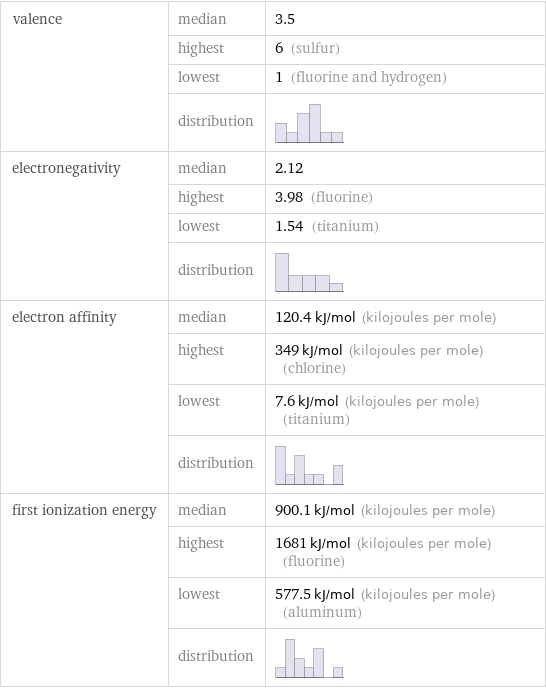
valence | median | 3.5 | highest | 6 (sulfur) | lowest | 1 (fluorine and hydrogen) | distribution | electronegativity | median | 2.12 | highest | 3.98 (fluorine) | lowest | 1.54 (titanium) | distribution | electron affinity | median | 120.4 kJ/mol (kilojoules per mole) | highest | 349 kJ/mol (kilojoules per mole) (chlorine) | lowest | 7.6 kJ/mol (kilojoules per mole) (titanium) | distribution | first ionization energy | median | 900.1 kJ/mol (kilojoules per mole) | highest | 1681 kJ/mol (kilojoules per mole) (fluorine) | lowest | 577.5 kJ/mol (kilojoules per mole) (aluminum) | distribution |
Atomic properties
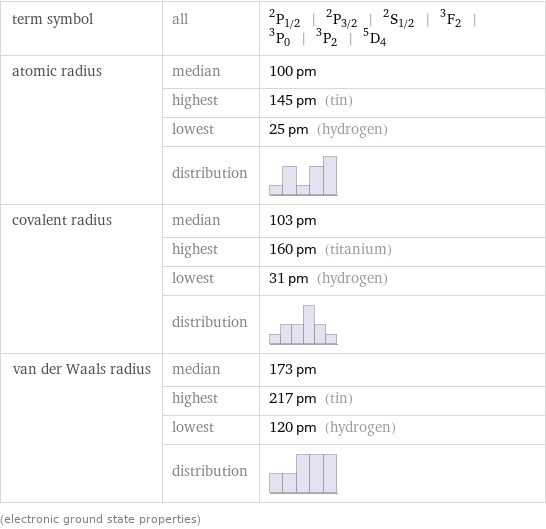
term symbol | all | ^2P_(1/2) | ^2P_(3/2) | ^2S_(1/2) | ^3F_2 | ^3P_0 | ^3P_2 | ^5D_4 atomic radius | median | 100 pm | highest | 145 pm (tin) | lowest | 25 pm (hydrogen) | distribution | covalent radius | median | 103 pm | highest | 160 pm (titanium) | lowest | 31 pm (hydrogen) | distribution | van der Waals radius | median | 173 pm | highest | 217 pm (tin) | lowest | 120 pm (hydrogen) | distribution | (electronic ground state properties)