Input interpretation
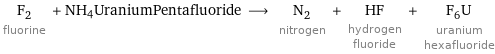
F_2 fluorine + NH4UraniumPentafluoride ⟶ N_2 nitrogen + HF hydrogen fluoride + F_6U uranium hexafluoride
Sample reactions
(data not available)
Chemical names and formulas
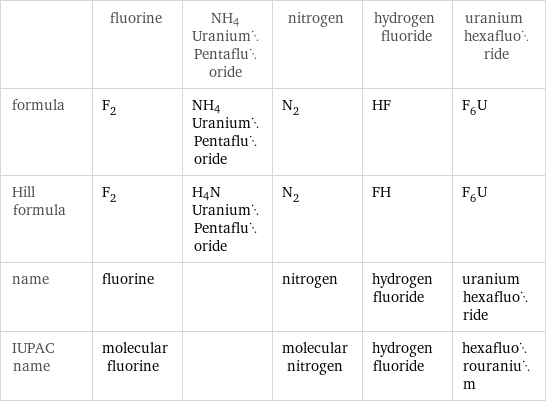
| fluorine | NH4UraniumPentafluoride | nitrogen | hydrogen fluoride | uranium hexafluoride formula | F_2 | NH4UraniumPentafluoride | N_2 | HF | F_6U Hill formula | F_2 | H4NUraniumPentafluoride | N_2 | FH | F_6U name | fluorine | | nitrogen | hydrogen fluoride | uranium hexafluoride IUPAC name | molecular fluorine | | molecular nitrogen | hydrogen fluoride | hexafluorouranium
Substance properties
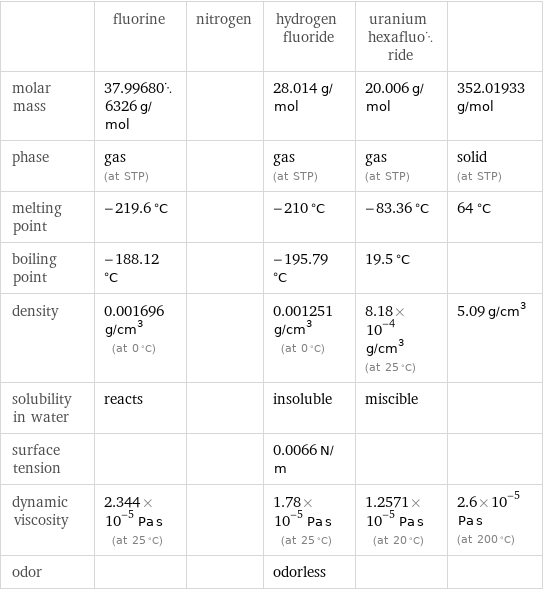
| fluorine | nitrogen | hydrogen fluoride | uranium hexafluoride | molar mass | 37.996806326 g/mol | | 28.014 g/mol | 20.006 g/mol | 352.01933 g/mol phase | gas (at STP) | | gas (at STP) | gas (at STP) | solid (at STP) melting point | -219.6 °C | | -210 °C | -83.36 °C | 64 °C boiling point | -188.12 °C | | -195.79 °C | 19.5 °C | density | 0.001696 g/cm^3 (at 0 °C) | | 0.001251 g/cm^3 (at 0 °C) | 8.18×10^-4 g/cm^3 (at 25 °C) | 5.09 g/cm^3 solubility in water | reacts | | insoluble | miscible | surface tension | | | 0.0066 N/m | | dynamic viscosity | 2.344×10^-5 Pa s (at 25 °C) | | 1.78×10^-5 Pa s (at 25 °C) | 1.2571×10^-5 Pa s (at 20 °C) | 2.6×10^-5 Pa s (at 200 °C) odor | | | odorless | |
Units