Input interpretation
sodium bromate | (S)-2-chloropropionic acid
Chemical names and formulas
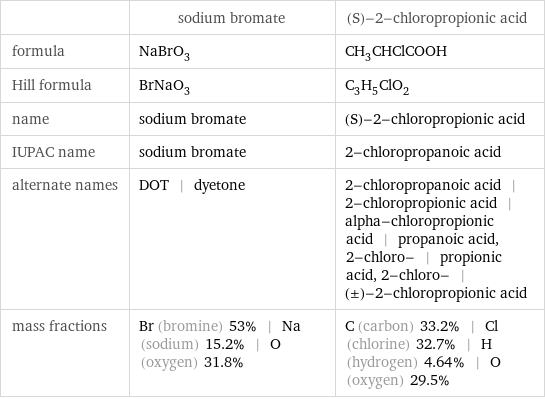
| sodium bromate | (S)-2-chloropropionic acid formula | NaBrO_3 | CH_3CHClCOOH Hill formula | BrNaO_3 | C_3H_5ClO_2 name | sodium bromate | (S)-2-chloropropionic acid IUPAC name | sodium bromate | 2-chloropropanoic acid alternate names | DOT | dyetone | 2-chloropropanoic acid | 2-chloropropionic acid | alpha-chloropropionic acid | propanoic acid, 2-chloro- | propionic acid, 2-chloro- | (±)-2-chloropropionic acid mass fractions | Br (bromine) 53% | Na (sodium) 15.2% | O (oxygen) 31.8% | C (carbon) 33.2% | Cl (chlorine) 32.7% | H (hydrogen) 4.64% | O (oxygen) 29.5%
Structure diagrams
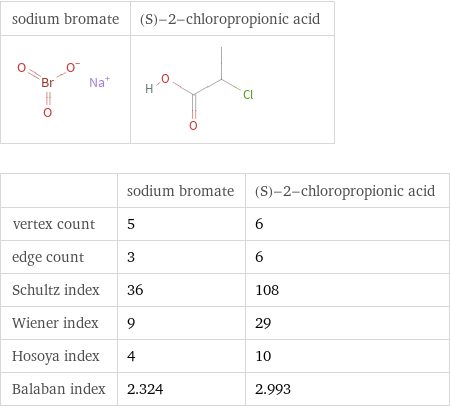
| sodium bromate | (S)-2-chloropropionic acid vertex count | 5 | 6 edge count | 3 | 6 Schultz index | 36 | 108 Wiener index | 9 | 29 Hosoya index | 4 | 10 Balaban index | 2.324 | 2.993
3D structure
3D structure
Basic properties
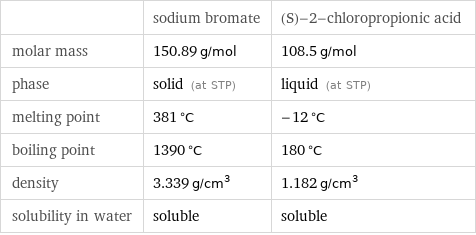
| sodium bromate | (S)-2-chloropropionic acid molar mass | 150.89 g/mol | 108.5 g/mol phase | solid (at STP) | liquid (at STP) melting point | 381 °C | -12 °C boiling point | 1390 °C | 180 °C density | 3.339 g/cm^3 | 1.182 g/cm^3 solubility in water | soluble | soluble
Units

Liquid properties
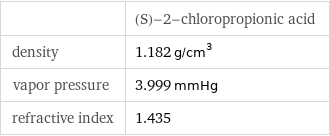
| (S)-2-chloropropionic acid density | 1.182 g/cm^3 vapor pressure | 3.999 mmHg refractive index | 1.435
Units

Thermodynamic properties
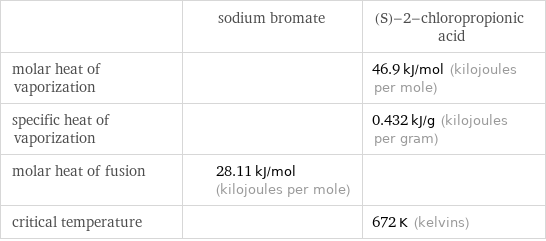
| sodium bromate | (S)-2-chloropropionic acid molar heat of vaporization | | 46.9 kJ/mol (kilojoules per mole) specific heat of vaporization | | 0.432 kJ/g (kilojoules per gram) molar heat of fusion | 28.11 kJ/mol (kilojoules per mole) | critical temperature | | 672 K (kelvins)
Solid properties
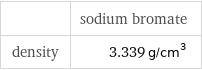
| sodium bromate density | 3.339 g/cm^3
Units

Chemical identifiers
![| sodium bromate | (S)-2-chloropropionic acid CAS number | 7789-38-0 | 598-78-7 Beilstein number | | 1720259 PubChem CID number | 23668195 | 11734 PubChem SID number | 24853461 | 24846904 SMILES identifier | [O-]Br(=O)=O.[Na+] | CC(C(=O)O)Cl](../image_source/563053e03a1a373a0bfc49b550732500.png)
| sodium bromate | (S)-2-chloropropionic acid CAS number | 7789-38-0 | 598-78-7 Beilstein number | | 1720259 PubChem CID number | 23668195 | 11734 PubChem SID number | 24853461 | 24846904 SMILES identifier | [O-]Br(=O)=O.[Na+] | CC(C(=O)O)Cl