Input interpretation
phosphinic acid | calcium plumbate
Chemical names and formulas
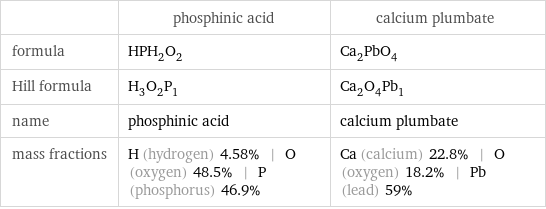
| phosphinic acid | calcium plumbate formula | HPH_2O_2 | Ca_2PbO_4 Hill formula | H_3O_2P_1 | Ca_2O_4Pb_1 name | phosphinic acid | calcium plumbate mass fractions | H (hydrogen) 4.58% | O (oxygen) 48.5% | P (phosphorus) 46.9% | Ca (calcium) 22.8% | O (oxygen) 18.2% | Pb (lead) 59%
Structure diagrams
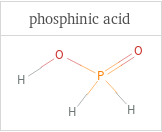
Structure diagrams
Basic properties
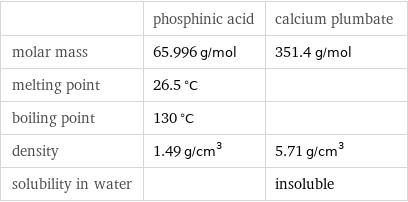
| phosphinic acid | calcium plumbate molar mass | 65.996 g/mol | 351.4 g/mol melting point | 26.5 °C | boiling point | 130 °C | density | 1.49 g/cm^3 | 5.71 g/cm^3 solubility in water | | insoluble
Units
