Input interpretation

diatomic molecules | molar mass
Summary
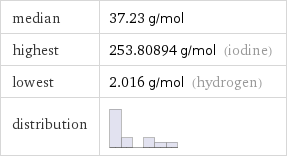
median | 37.23 g/mol highest | 253.80894 g/mol (iodine) lowest | 2.016 g/mol (hydrogen) distribution |
Units

Distribution plots
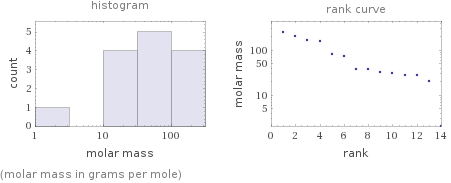
(molar mass in grams per mole)
Molar mass rankings
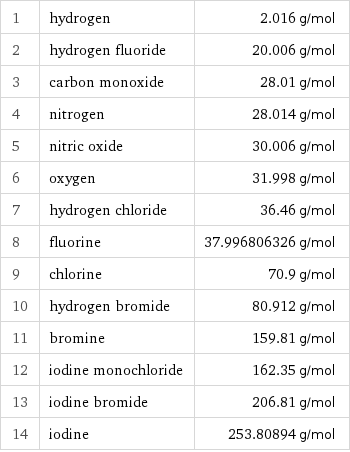
1 | hydrogen | 2.016 g/mol 2 | hydrogen fluoride | 20.006 g/mol 3 | carbon monoxide | 28.01 g/mol 4 | nitrogen | 28.014 g/mol 5 | nitric oxide | 30.006 g/mol 6 | oxygen | 31.998 g/mol 7 | hydrogen chloride | 36.46 g/mol 8 | fluorine | 37.996806326 g/mol 9 | chlorine | 70.9 g/mol 10 | hydrogen bromide | 80.912 g/mol 11 | bromine | 159.81 g/mol 12 | iodine monochloride | 162.35 g/mol 13 | iodine bromide | 206.81 g/mol 14 | iodine | 253.80894 g/mol
Unit conversion for median molar mass 37.23 g/mol
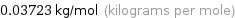
0.03723 kg/mol (kilograms per mole)
Comparisons for median molar mass 37.23 g/mol
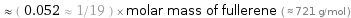
≈ ( 0.052 ≈ 1/19 ) × molar mass of fullerene ( ≈ 721 g/mol )
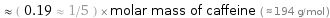
≈ ( 0.19 ≈ 1/5 ) × molar mass of caffeine ( ≈ 194 g/mol )
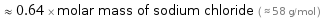
≈ 0.64 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
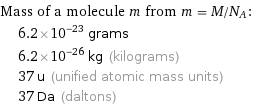
Mass of a molecule m from m = M/N_A: | 6.2×10^-23 grams | 6.2×10^-26 kg (kilograms) | 37 u (unified atomic mass units) | 37 Da (daltons)
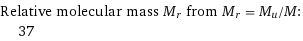
Relative molecular mass M_r from M_r = M_u/M: | 37