Input interpretation
lithium fluoride | trifluoromethanesulfonic acid
Chemical names and formulas
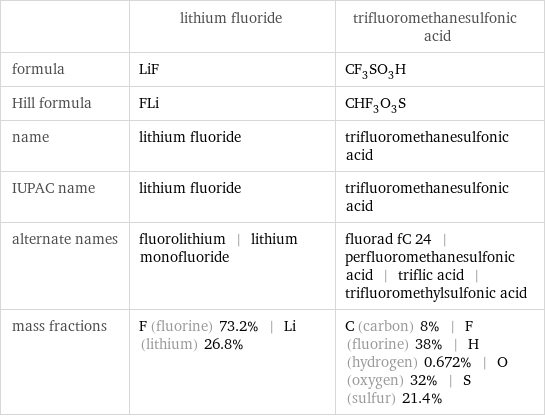
| lithium fluoride | trifluoromethanesulfonic acid formula | LiF | CF_3SO_3H Hill formula | FLi | CHF_3O_3S name | lithium fluoride | trifluoromethanesulfonic acid IUPAC name | lithium fluoride | trifluoromethanesulfonic acid alternate names | fluorolithium | lithium monofluoride | fluorad fC 24 | perfluoromethanesulfonic acid | triflic acid | trifluoromethylsulfonic acid mass fractions | F (fluorine) 73.2% | Li (lithium) 26.8% | C (carbon) 8% | F (fluorine) 38% | H (hydrogen) 0.672% | O (oxygen) 32% | S (sulfur) 21.4%
Structure diagrams
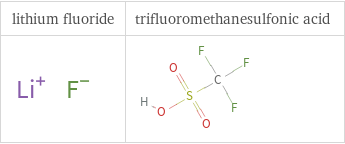
Structure diagrams
3D structure
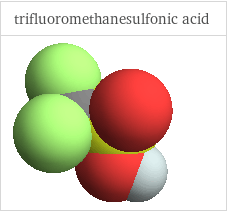
3D structure
Basic properties
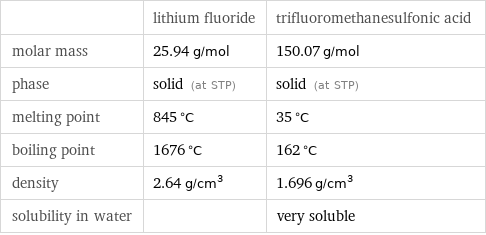
| lithium fluoride | trifluoromethanesulfonic acid molar mass | 25.94 g/mol | 150.07 g/mol phase | solid (at STP) | solid (at STP) melting point | 845 °C | 35 °C boiling point | 1676 °C | 162 °C density | 2.64 g/cm^3 | 1.696 g/cm^3 solubility in water | | very soluble
Units

Thermodynamic properties
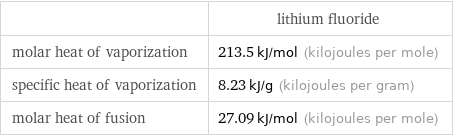
| lithium fluoride molar heat of vaporization | 213.5 kJ/mol (kilojoules per mole) specific heat of vaporization | 8.23 kJ/g (kilojoules per gram) molar heat of fusion | 27.09 kJ/mol (kilojoules per mole)
Solid properties (at STP)
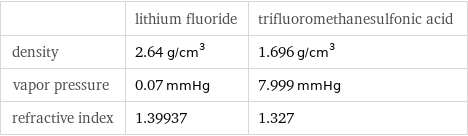
| lithium fluoride | trifluoromethanesulfonic acid density | 2.64 g/cm^3 | 1.696 g/cm^3 vapor pressure | 0.07 mmHg | 7.999 mmHg refractive index | 1.39937 | 1.327
Units
