Input interpretation

weak electrolytes | boiling point
Summary
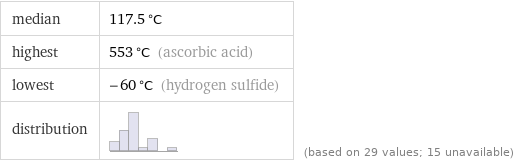
median | 117.5 °C highest | 553 °C (ascorbic acid) lowest | -60 °C (hydrogen sulfide) distribution | | (based on 29 values; 15 unavailable)
Entities with missing values
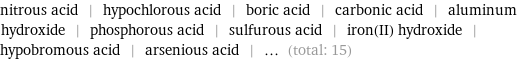
nitrous acid | hypochlorous acid | boric acid | carbonic acid | aluminum hydroxide | phosphorous acid | sulfurous acid | iron(II) hydroxide | hypobromous acid | arsenious acid | ... (total: 15)
Distribution plots
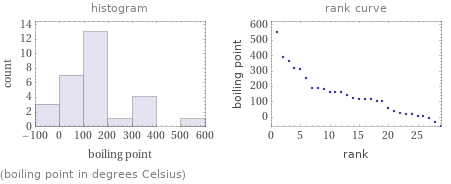
(boiling point in degrees Celsius)
Boiling point rankings
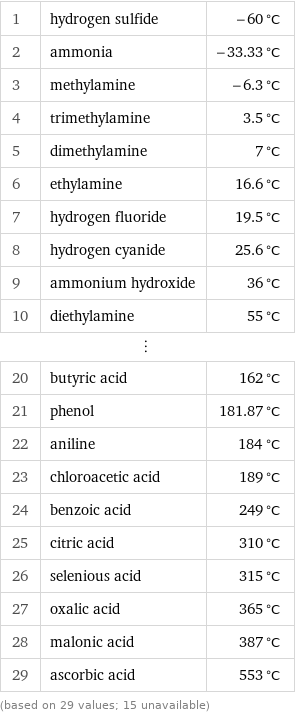
1 | hydrogen sulfide | -60 °C 2 | ammonia | -33.33 °C 3 | methylamine | -6.3 °C 4 | trimethylamine | 3.5 °C 5 | dimethylamine | 7 °C 6 | ethylamine | 16.6 °C 7 | hydrogen fluoride | 19.5 °C 8 | hydrogen cyanide | 25.6 °C 9 | ammonium hydroxide | 36 °C 10 | diethylamine | 55 °C ⋮ | | 20 | butyric acid | 162 °C 21 | phenol | 181.87 °C 22 | aniline | 184 °C 23 | chloroacetic acid | 189 °C 24 | benzoic acid | 249 °C 25 | citric acid | 310 °C 26 | selenious acid | 315 °C 27 | oxalic acid | 365 °C 28 | malonic acid | 387 °C 29 | ascorbic acid | 553 °C (based on 29 values; 15 unavailable)
Unit conversions for median boiling point 117.5 °C
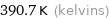
390.7 K (kelvins)

243.5 °F (degrees Fahrenheit)
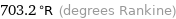
703.2 °R (degrees Rankine)
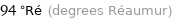
94 °Ré (degrees Réaumur)

69.19 °Rø (degrees Rømer)
Comparison for median boiling point 117.5 °C
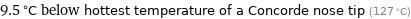
9.5 °C below hottest temperature of a Concorde nose tip (127 °C)
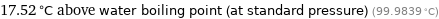
17.52 °C above water boiling point (at standard pressure) (99.9839 °C)
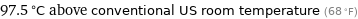
97.5 °C above conventional US room temperature (68 °F)
Corresponding quantities

Thermodynamic energy E from E = kT: | 34 meV (millielectronvolts)
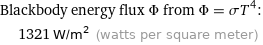
Blackbody energy flux Φ from Φ = σT^4: | 1321 W/m^2 (watts per square meter)

Approximate luminous exitance from a planar blackbody radiator perpendicular to its surface: | 1.1×10^-14 lx (lux)
Nearest corresponding gas marks for median boiling point 117.5 °C (degrees Celsius)

| temperature | usage gas mark 1/2 | 120 °C | United Kingdom thermostat 4 | 120 °C | France (actual measurements may vary)