Input interpretation
strontium hydride | potassium hexacyanoferrate(III)
Chemical names and formulas
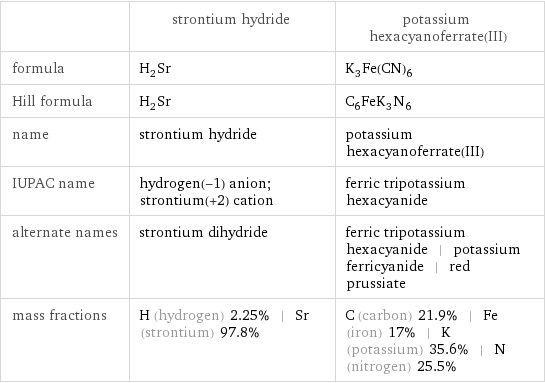
| strontium hydride | potassium hexacyanoferrate(III) formula | H_2Sr | K_3Fe(CN)_6 Hill formula | H_2Sr | C_6FeK_3N_6 name | strontium hydride | potassium hexacyanoferrate(III) IUPAC name | hydrogen(-1) anion; strontium(+2) cation | ferric tripotassium hexacyanide alternate names | strontium dihydride | ferric tripotassium hexacyanide | potassium ferricyanide | red prussiate mass fractions | H (hydrogen) 2.25% | Sr (strontium) 97.8% | C (carbon) 21.9% | Fe (iron) 17% | K (potassium) 35.6% | N (nitrogen) 25.5%
Structure diagrams
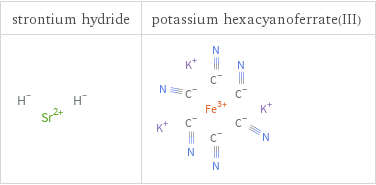
Structure diagrams
Basic properties
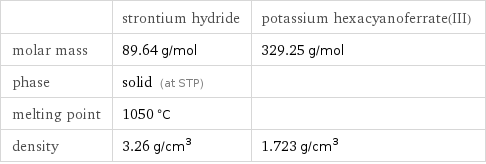
| strontium hydride | potassium hexacyanoferrate(III) molar mass | 89.64 g/mol | 329.25 g/mol phase | solid (at STP) | melting point | 1050 °C | density | 3.26 g/cm^3 | 1.723 g/cm^3
Units

Solid properties
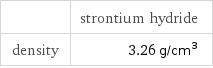
| strontium hydride density | 3.26 g/cm^3
Units
