Input interpretation
potassium iodate | iron
Chemical names and formulas
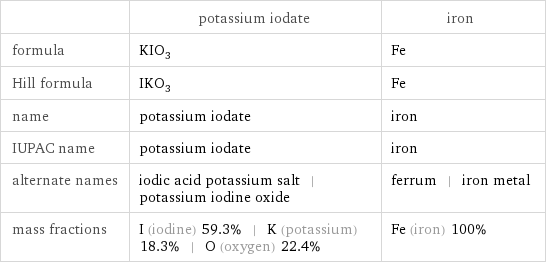
| potassium iodate | iron formula | KIO_3 | Fe Hill formula | IKO_3 | Fe name | potassium iodate | iron IUPAC name | potassium iodate | iron alternate names | iodic acid potassium salt | potassium iodine oxide | ferrum | iron metal mass fractions | I (iodine) 59.3% | K (potassium) 18.3% | O (oxygen) 22.4% | Fe (iron) 100%
Structure diagrams

Structure diagrams
Basic properties
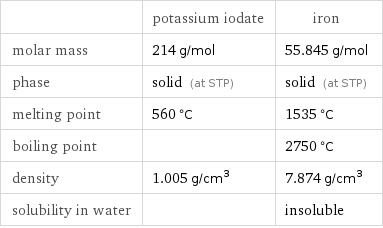
| potassium iodate | iron molar mass | 214 g/mol | 55.845 g/mol phase | solid (at STP) | solid (at STP) melting point | 560 °C | 1535 °C boiling point | | 2750 °C density | 1.005 g/cm^3 | 7.874 g/cm^3 solubility in water | | insoluble
Units
