Input interpretation
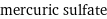
mercuric sulfate
Chemical names and formulas

formula | HgSO_4 Hill formula | HgO_4S name | mercuric sulfate IUPAC name | mercury(+2) cation sulfate alternate names | mercuric sulphate | mercury bisulfate | mercury(II) sulfate | mercury(II) sulphate mass fractions | Hg (mercury) 67.6% | O (oxygen) 21.6% | S (sulfur) 10.8%
Structure diagram
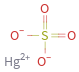
Structure diagram

vertex count | 6 edge count | 4 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties

molar mass | 296.65 g/mol phase | solid (at STP) melting point | 850 °C density | 5.995 g/cm^3 solubility in water | decomposes
Units

Solid properties (at STP)
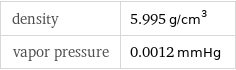
density | 5.995 g/cm^3 vapor pressure | 0.0012 mmHg
Units

Thermodynamic properties
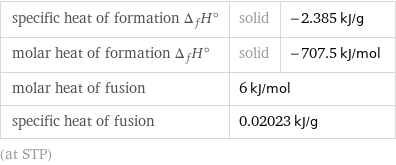
specific heat of formation Δ_fH° | solid | -2.385 kJ/g molar heat of formation Δ_fH° | solid | -707.5 kJ/mol molar heat of fusion | 6 kJ/mol | specific heat of fusion | 0.02023 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7783-35-9 PubChem CID number | 24544 SMILES identifier | [O-]S(=O)(=O)[O-].[Hg+2] InChI identifier | InChI=1/Hg.H2O4S/c;1-5(2, 3)4/h;(H2, 1, 2, 3, 4)/q+2;/p-2/fHg.O4S/qm;-2 EU number | 231-992-5 Gmelin number | 32386 RTECS number | OX0500000](../image_source/1e53c54f53a163d81f3ba765c4e689e2.png)
CAS number | 7783-35-9 PubChem CID number | 24544 SMILES identifier | [O-]S(=O)(=O)[O-].[Hg+2] InChI identifier | InChI=1/Hg.H2O4S/c;1-5(2, 3)4/h;(H2, 1, 2, 3, 4)/q+2;/p-2/fHg.O4S/qm;-2 EU number | 231-992-5 Gmelin number | 32386 RTECS number | OX0500000
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 0 NFPA reactivity rating | 0
Safety properties

autoignition point | 450 °C

DOT hazard class | 6.1 DOT numbers | 1645
Toxicity properties

RTECS classes | other
Ion equivalents

Hg^(2+) (mercury(II) cation) | 1 (SO_4)^(2-) (sulfate anion) | 1