Input interpretation
vanadium (chemical element) | cobalt (chemical element) | chromium (chemical element)
Periodic table location
Periodic table location
Images
Images
Basic elemental properties
![| vanadium | cobalt | chromium atomic symbol | V | Co | Cr atomic number | 23 | 27 | 24 short electronic configuration | [Ar]4s^23d^3 | [Ar]4s^23d^7 | [Ar]4s^13d^5 Aufbau diagram | 3d 4s | 3d 4s | 3d 4s block | d | d | d group | 5 | 9 | 6 period | 4 | 4 | 4 atomic mass | 50.9415 u | 58.933194 u | 51.9961 u half-life | (stable) | (stable) | (stable)](../image_source/a39bccbee87c892543ac5a5d33c045ff.png)
| vanadium | cobalt | chromium atomic symbol | V | Co | Cr atomic number | 23 | 27 | 24 short electronic configuration | [Ar]4s^23d^3 | [Ar]4s^23d^7 | [Ar]4s^13d^5 Aufbau diagram | 3d 4s | 3d 4s | 3d 4s block | d | d | d group | 5 | 9 | 6 period | 4 | 4 | 4 atomic mass | 50.9415 u | 58.933194 u | 51.9961 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
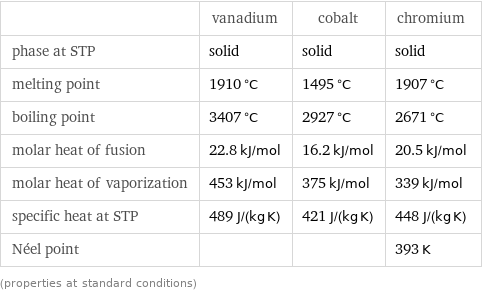
| vanadium | cobalt | chromium phase at STP | solid | solid | solid melting point | 1910 °C | 1495 °C | 1907 °C boiling point | 3407 °C | 2927 °C | 2671 °C molar heat of fusion | 22.8 kJ/mol | 16.2 kJ/mol | 20.5 kJ/mol molar heat of vaporization | 453 kJ/mol | 375 kJ/mol | 339 kJ/mol specific heat at STP | 489 J/(kg K) | 421 J/(kg K) | 448 J/(kg K) Néel point | | | 393 K (properties at standard conditions)
Material properties
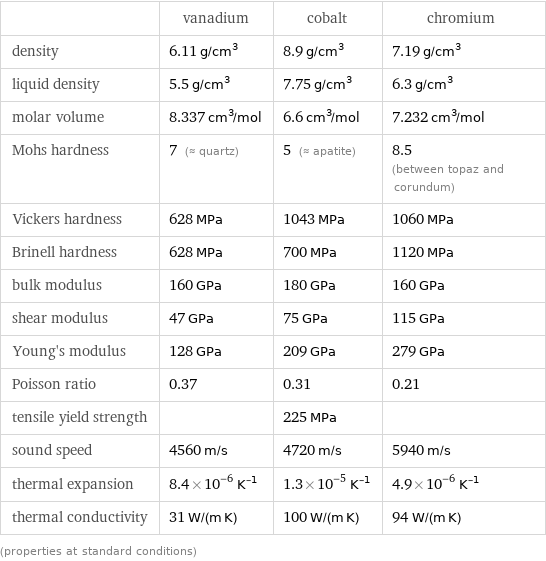
| vanadium | cobalt | chromium density | 6.11 g/cm^3 | 8.9 g/cm^3 | 7.19 g/cm^3 liquid density | 5.5 g/cm^3 | 7.75 g/cm^3 | 6.3 g/cm^3 molar volume | 8.337 cm^3/mol | 6.6 cm^3/mol | 7.232 cm^3/mol Mohs hardness | 7 (≈ quartz) | 5 (≈ apatite) | 8.5 (between topaz and corundum) Vickers hardness | 628 MPa | 1043 MPa | 1060 MPa Brinell hardness | 628 MPa | 700 MPa | 1120 MPa bulk modulus | 160 GPa | 180 GPa | 160 GPa shear modulus | 47 GPa | 75 GPa | 115 GPa Young's modulus | 128 GPa | 209 GPa | 279 GPa Poisson ratio | 0.37 | 0.31 | 0.21 tensile yield strength | | 225 MPa | sound speed | 4560 m/s | 4720 m/s | 5940 m/s thermal expansion | 8.4×10^-6 K^(-1) | 1.3×10^-5 K^(-1) | 4.9×10^-6 K^(-1) thermal conductivity | 31 W/(m K) | 100 W/(m K) | 94 W/(m K) (properties at standard conditions)
Electromagnetic properties
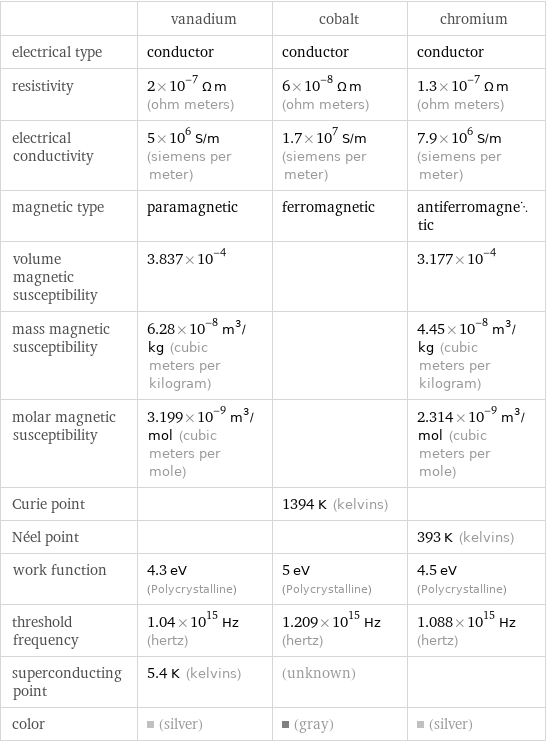
| vanadium | cobalt | chromium electrical type | conductor | conductor | conductor resistivity | 2×10^-7 Ω m (ohm meters) | 6×10^-8 Ω m (ohm meters) | 1.3×10^-7 Ω m (ohm meters) electrical conductivity | 5×10^6 S/m (siemens per meter) | 1.7×10^7 S/m (siemens per meter) | 7.9×10^6 S/m (siemens per meter) magnetic type | paramagnetic | ferromagnetic | antiferromagnetic volume magnetic susceptibility | 3.837×10^-4 | | 3.177×10^-4 mass magnetic susceptibility | 6.28×10^-8 m^3/kg (cubic meters per kilogram) | | 4.45×10^-8 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | 3.199×10^-9 m^3/mol (cubic meters per mole) | | 2.314×10^-9 m^3/mol (cubic meters per mole) Curie point | | 1394 K (kelvins) | Néel point | | | 393 K (kelvins) work function | 4.3 eV (Polycrystalline) | 5 eV (Polycrystalline) | 4.5 eV (Polycrystalline) threshold frequency | 1.04×10^15 Hz (hertz) | 1.209×10^15 Hz (hertz) | 1.088×10^15 Hz (hertz) superconducting point | 5.4 K (kelvins) | (unknown) | color | (silver) | (gray) | (silver)
Reactivity
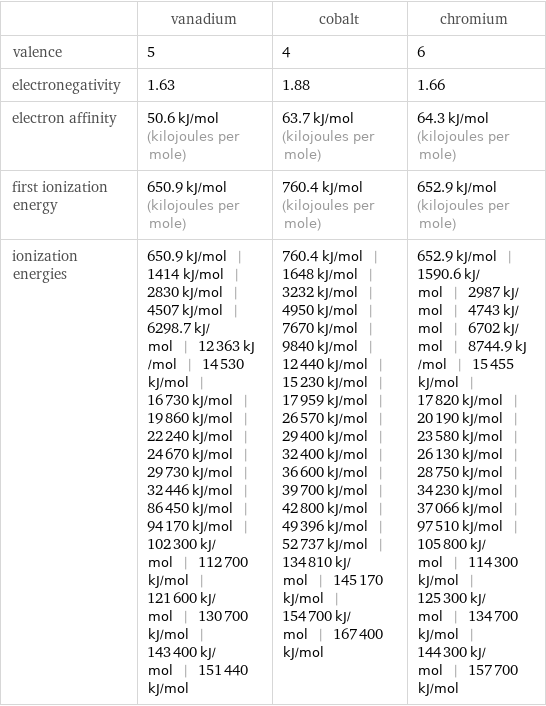
| vanadium | cobalt | chromium valence | 5 | 4 | 6 electronegativity | 1.63 | 1.88 | 1.66 electron affinity | 50.6 kJ/mol (kilojoules per mole) | 63.7 kJ/mol (kilojoules per mole) | 64.3 kJ/mol (kilojoules per mole) first ionization energy | 650.9 kJ/mol (kilojoules per mole) | 760.4 kJ/mol (kilojoules per mole) | 652.9 kJ/mol (kilojoules per mole) ionization energies | 650.9 kJ/mol | 1414 kJ/mol | 2830 kJ/mol | 4507 kJ/mol | 6298.7 kJ/mol | 12363 kJ/mol | 14530 kJ/mol | 16730 kJ/mol | 19860 kJ/mol | 22240 kJ/mol | 24670 kJ/mol | 29730 kJ/mol | 32446 kJ/mol | 86450 kJ/mol | 94170 kJ/mol | 102300 kJ/mol | 112700 kJ/mol | 121600 kJ/mol | 130700 kJ/mol | 143400 kJ/mol | 151440 kJ/mol | 760.4 kJ/mol | 1648 kJ/mol | 3232 kJ/mol | 4950 kJ/mol | 7670 kJ/mol | 9840 kJ/mol | 12440 kJ/mol | 15230 kJ/mol | 17959 kJ/mol | 26570 kJ/mol | 29400 kJ/mol | 32400 kJ/mol | 36600 kJ/mol | 39700 kJ/mol | 42800 kJ/mol | 49396 kJ/mol | 52737 kJ/mol | 134810 kJ/mol | 145170 kJ/mol | 154700 kJ/mol | 167400 kJ/mol | 652.9 kJ/mol | 1590.6 kJ/mol | 2987 kJ/mol | 4743 kJ/mol | 6702 kJ/mol | 8744.9 kJ/mol | 15455 kJ/mol | 17820 kJ/mol | 20190 kJ/mol | 23580 kJ/mol | 26130 kJ/mol | 28750 kJ/mol | 34230 kJ/mol | 37066 kJ/mol | 97510 kJ/mol | 105800 kJ/mol | 114300 kJ/mol | 125300 kJ/mol | 134700 kJ/mol | 144300 kJ/mol | 157700 kJ/mol
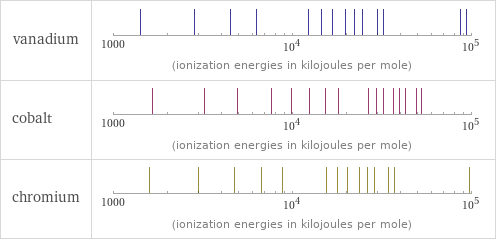
Reactivity
Atomic properties
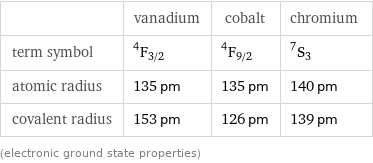
| vanadium | cobalt | chromium term symbol | ^4F_(3/2) | ^4F_(9/2) | ^7S_3 atomic radius | 135 pm | 135 pm | 140 pm covalent radius | 153 pm | 126 pm | 139 pm (electronic ground state properties)
Abundances
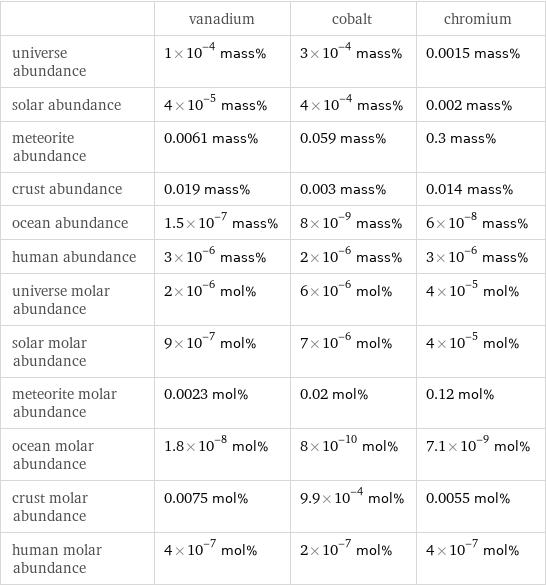
| vanadium | cobalt | chromium universe abundance | 1×10^-4 mass% | 3×10^-4 mass% | 0.0015 mass% solar abundance | 4×10^-5 mass% | 4×10^-4 mass% | 0.002 mass% meteorite abundance | 0.0061 mass% | 0.059 mass% | 0.3 mass% crust abundance | 0.019 mass% | 0.003 mass% | 0.014 mass% ocean abundance | 1.5×10^-7 mass% | 8×10^-9 mass% | 6×10^-8 mass% human abundance | 3×10^-6 mass% | 2×10^-6 mass% | 3×10^-6 mass% universe molar abundance | 2×10^-6 mol% | 6×10^-6 mol% | 4×10^-5 mol% solar molar abundance | 9×10^-7 mol% | 7×10^-6 mol% | 4×10^-5 mol% meteorite molar abundance | 0.0023 mol% | 0.02 mol% | 0.12 mol% ocean molar abundance | 1.8×10^-8 mol% | 8×10^-10 mol% | 7.1×10^-9 mol% crust molar abundance | 0.0075 mol% | 9.9×10^-4 mol% | 0.0055 mol% human molar abundance | 4×10^-7 mol% | 2×10^-7 mol% | 4×10^-7 mol%