Input interpretation
stannic chloride pentahydrate | lutetium sulfide
Chemical names and formulas
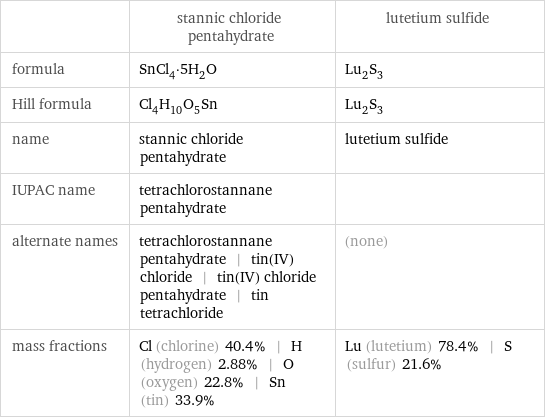
| stannic chloride pentahydrate | lutetium sulfide formula | SnCl_4·5H_2O | Lu_2S_3 Hill formula | Cl_4H_10O_5Sn | Lu_2S_3 name | stannic chloride pentahydrate | lutetium sulfide IUPAC name | tetrachlorostannane pentahydrate | alternate names | tetrachlorostannane pentahydrate | tin(IV) chloride | tin(IV) chloride pentahydrate | tin tetrachloride | (none) mass fractions | Cl (chlorine) 40.4% | H (hydrogen) 2.88% | O (oxygen) 22.8% | Sn (tin) 33.9% | Lu (lutetium) 78.4% | S (sulfur) 21.6%
Structure diagrams
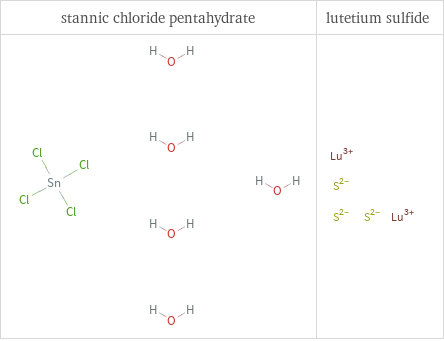
Structure diagrams
Basic properties

| stannic chloride pentahydrate | lutetium sulfide molar mass | 350.6 g/mol | 446.11 g/mol density | 2.2 g/cm^3 | 6.26 g/cm^3 solubility in water | soluble |
Units
