Input interpretation
neodymium(III) sulfate octahydrate | hypochlorous acid
Chemical names and formulas
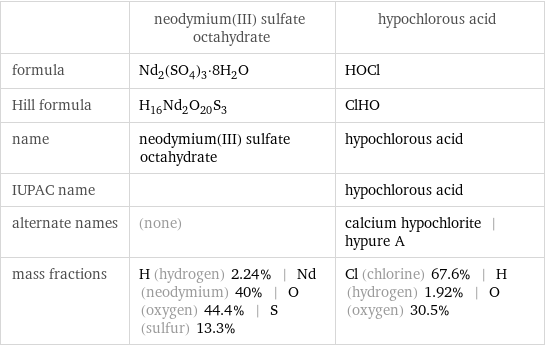
| neodymium(III) sulfate octahydrate | hypochlorous acid formula | Nd_2(SO_4)_3·8H_2O | HOCl Hill formula | H_16Nd_2O_20S_3 | ClHO name | neodymium(III) sulfate octahydrate | hypochlorous acid IUPAC name | | hypochlorous acid alternate names | (none) | calcium hypochlorite | hypure A mass fractions | H (hydrogen) 2.24% | Nd (neodymium) 40% | O (oxygen) 44.4% | S (sulfur) 13.3% | Cl (chlorine) 67.6% | H (hydrogen) 1.92% | O (oxygen) 30.5%
Structure diagrams
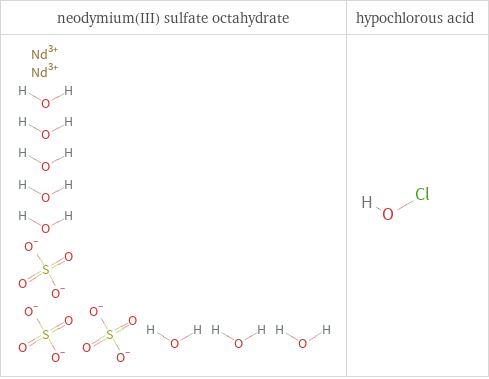
Structure diagrams
3D structure
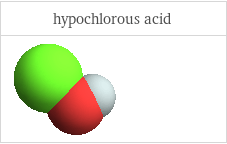
3D structure
Basic properties

| neodymium(III) sulfate octahydrate | hypochlorous acid molar mass | 720.8 g/mol | 52.46 g/mol density | 2.85 g/cm^3 | solubility in water | slightly soluble | soluble
Units
