Input interpretation
sodium bromate | phosphorus pentachloride
Chemical names and formulas
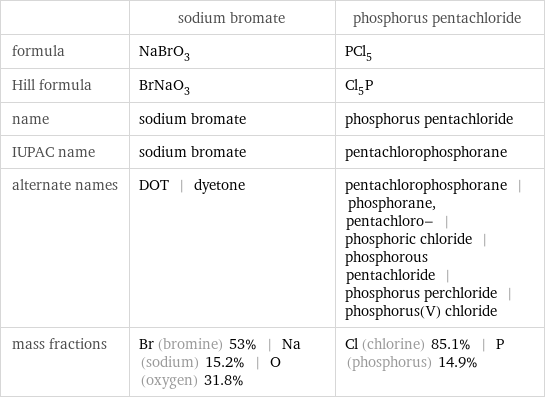
| sodium bromate | phosphorus pentachloride formula | NaBrO_3 | PCl_5 Hill formula | BrNaO_3 | Cl_5P name | sodium bromate | phosphorus pentachloride IUPAC name | sodium bromate | pentachlorophosphorane alternate names | DOT | dyetone | pentachlorophosphorane | phosphorane, pentachloro- | phosphoric chloride | phosphorous pentachloride | phosphorus perchloride | phosphorus(V) chloride mass fractions | Br (bromine) 53% | Na (sodium) 15.2% | O (oxygen) 31.8% | Cl (chlorine) 85.1% | P (phosphorus) 14.9%
Structure diagrams
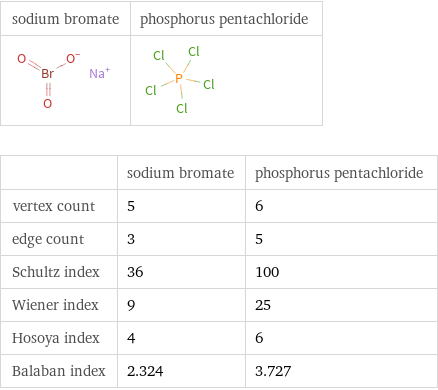
| sodium bromate | phosphorus pentachloride vertex count | 5 | 6 edge count | 3 | 5 Schultz index | 36 | 100 Wiener index | 9 | 25 Hosoya index | 4 | 6 Balaban index | 2.324 | 3.727
Basic properties
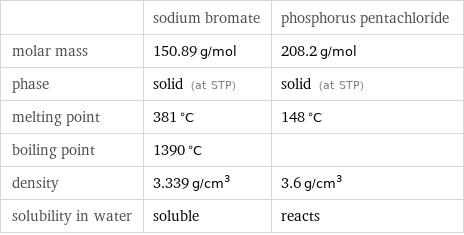
| sodium bromate | phosphorus pentachloride molar mass | 150.89 g/mol | 208.2 g/mol phase | solid (at STP) | solid (at STP) melting point | 381 °C | 148 °C boiling point | 1390 °C | density | 3.339 g/cm^3 | 3.6 g/cm^3 solubility in water | soluble | reacts
Units

Thermodynamic properties
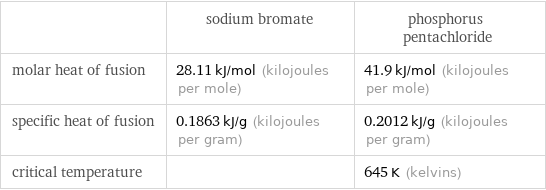
| sodium bromate | phosphorus pentachloride molar heat of fusion | 28.11 kJ/mol (kilojoules per mole) | 41.9 kJ/mol (kilojoules per mole) specific heat of fusion | 0.1863 kJ/g (kilojoules per gram) | 0.2012 kJ/g (kilojoules per gram) critical temperature | | 645 K (kelvins)
Solid properties (at STP)

| sodium bromate | phosphorus pentachloride density | 3.339 g/cm^3 | 3.6 g/cm^3
Units

Chemical identifiers
![| sodium bromate | phosphorus pentachloride CAS number | 7789-38-0 | 10026-13-8 PubChem CID number | 23668195 | 24819 PubChem SID number | 24853461 | SMILES identifier | [O-]Br(=O)=O.[Na+] | P(Cl)(Cl)(Cl)(Cl)Cl InChI identifier | InChI=1/BrHO3.Na/c2-1(3)4;/h(H, 2, 3, 4);/q;+1/p-1/fBrO3.Na/q-1;m | InChI=1/Cl5P/c1-6(2, 3, 4)5 EU number | | 233-060-3 Gmelin number | | 2622 RTECS number | EF8750000 | TB6125000 MDL number | MFCD00003476 |](../image_source/362cd599b726b52139303124998ff851.png)
| sodium bromate | phosphorus pentachloride CAS number | 7789-38-0 | 10026-13-8 PubChem CID number | 23668195 | 24819 PubChem SID number | 24853461 | SMILES identifier | [O-]Br(=O)=O.[Na+] | P(Cl)(Cl)(Cl)(Cl)Cl InChI identifier | InChI=1/BrHO3.Na/c2-1(3)4;/h(H, 2, 3, 4);/q;+1/p-1/fBrO3.Na/q-1;m | InChI=1/Cl5P/c1-6(2, 3, 4)5 EU number | | 233-060-3 Gmelin number | | 2622 RTECS number | EF8750000 | TB6125000 MDL number | MFCD00003476 |
NFPA label

NFPA label
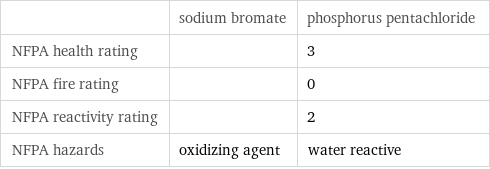
| sodium bromate | phosphorus pentachloride NFPA health rating | | 3 NFPA fire rating | | 0 NFPA reactivity rating | | 2 NFPA hazards | oxidizing agent | water reactive