Input interpretation
sulfate anion | permanganate anion | chromate anion
Structure diagrams
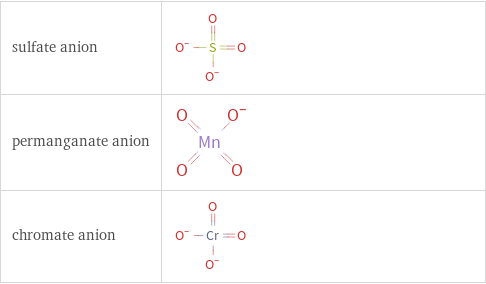
Structure diagrams
General properties
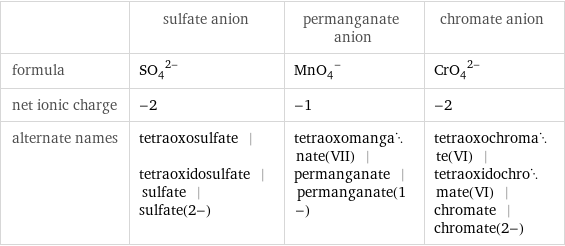
| sulfate anion | permanganate anion | chromate anion formula | (SO_4)^(2-) | (MnO_4)^- | (CrO_4)^(2-) net ionic charge | -2 | -1 | -2 alternate names | tetraoxosulfate | tetraoxidosulfate | sulfate | sulfate(2-) | tetraoxomanganate(VII) | permanganate | permanganate(1-) | tetraoxochromate(VI) | tetraoxidochromate(VI) | chromate | chromate(2-)
Ionic radii

| sulfate anion | permanganate anion | chromate anion thermochemical radius | 258 pm | 229 pm | 256 pm
Units

Other properties
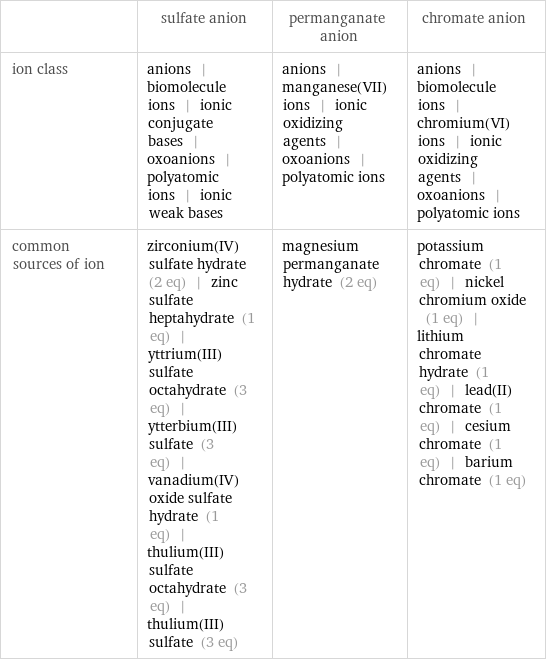
| sulfate anion | permanganate anion | chromate anion ion class | anions | biomolecule ions | ionic conjugate bases | oxoanions | polyatomic ions | ionic weak bases | anions | manganese(VII) ions | ionic oxidizing agents | oxoanions | polyatomic ions | anions | biomolecule ions | chromium(VI) ions | ionic oxidizing agents | oxoanions | polyatomic ions common sources of ion | zirconium(IV) sulfate hydrate (2 eq) | zinc sulfate heptahydrate (1 eq) | yttrium(III) sulfate octahydrate (3 eq) | ytterbium(III) sulfate (3 eq) | vanadium(IV) oxide sulfate hydrate (1 eq) | thulium(III) sulfate octahydrate (3 eq) | thulium(III) sulfate (3 eq) | magnesium permanganate hydrate (2 eq) | potassium chromate (1 eq) | nickel chromium oxide (1 eq) | lithium chromate hydrate (1 eq) | lead(II) chromate (1 eq) | cesium chromate (1 eq) | barium chromate (1 eq)