Input interpretation

weak electrolytes | Hill formula
Table
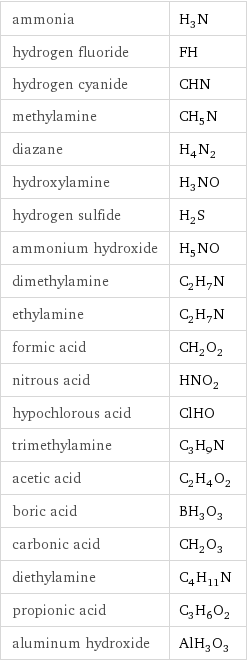
ammonia | H_3N hydrogen fluoride | FH hydrogen cyanide | CHN methylamine | CH_5N diazane | H_4N_2 hydroxylamine | H_3NO hydrogen sulfide | H_2S ammonium hydroxide | H_5NO dimethylamine | C_2H_7N ethylamine | C_2H_7N formic acid | CH_2O_2 nitrous acid | HNO_2 hypochlorous acid | ClHO trimethylamine | C_3H_9N acetic acid | C_2H_4O_2 boric acid | BH_3O_3 carbonic acid | CH_2O_3 diethylamine | C_4H_11N propionic acid | C_3H_6O_2 aluminum hydroxide | AlH_3O_3