Input interpretation
boric acid | quartz
Chemical names and formulas
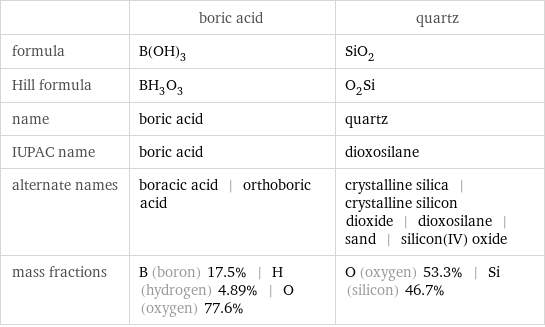
| boric acid | quartz formula | B(OH)_3 | SiO_2 Hill formula | BH_3O_3 | O_2Si name | boric acid | quartz IUPAC name | boric acid | dioxosilane alternate names | boracic acid | orthoboric acid | crystalline silica | crystalline silicon dioxide | dioxosilane | sand | silicon(IV) oxide mass fractions | B (boron) 17.5% | H (hydrogen) 4.89% | O (oxygen) 77.6% | O (oxygen) 53.3% | Si (silicon) 46.7%
Structure diagrams

| boric acid | quartz vertex count | 4 | 3 edge count | 6 | 2 Schultz index | 36 | 16 Wiener index | 9 | 4 Hosoya index | 4 | 3 Balaban index | 2.324 | 1.633
Basic properties
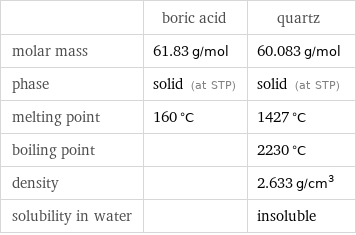
| boric acid | quartz molar mass | 61.83 g/mol | 60.083 g/mol phase | solid (at STP) | solid (at STP) melting point | 160 °C | 1427 °C boiling point | | 2230 °C density | | 2.633 g/cm^3 solubility in water | | insoluble
Units

Thermodynamic properties
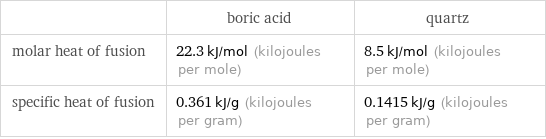
| boric acid | quartz molar heat of fusion | 22.3 kJ/mol (kilojoules per mole) | 8.5 kJ/mol (kilojoules per mole) specific heat of fusion | 0.361 kJ/g (kilojoules per gram) | 0.1415 kJ/g (kilojoules per gram)
Solid properties (at STP)

| boric acid | quartz density | | 2.633 g/cm^3 vapor pressure | 2.6 mmHg | 10 mmHg
Units
