Input interpretation

hexachloropalladate(IV) anion | ion class
Result

anions | complex ions | metal halide ion | palladium 4 ion
Ionic radii
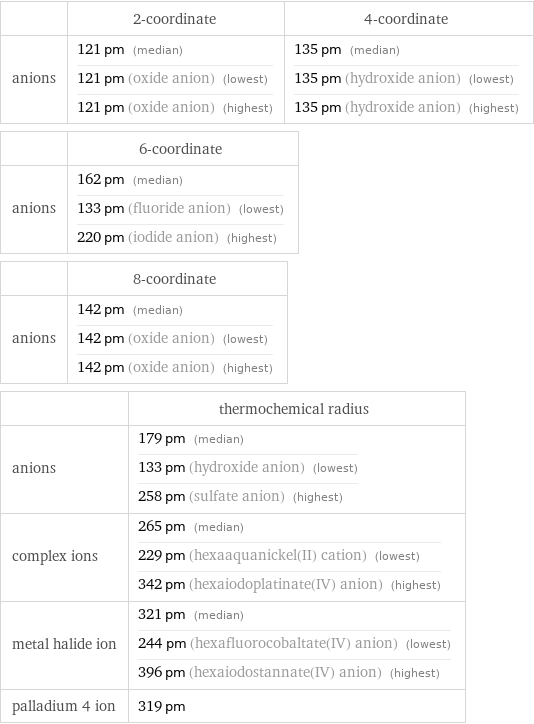
| 2-coordinate | 4-coordinate anions | 121 pm (median) 121 pm (oxide anion) (lowest) 121 pm (oxide anion) (highest) | 135 pm (median) 135 pm (hydroxide anion) (lowest) 135 pm (hydroxide anion) (highest) | 6-coordinate anions | 162 pm (median) 133 pm (fluoride anion) (lowest) 220 pm (iodide anion) (highest) | 8-coordinate anions | 142 pm (median) 142 pm (oxide anion) (lowest) 142 pm (oxide anion) (highest) | thermochemical radius anions | 179 pm (median) 133 pm (hydroxide anion) (lowest) 258 pm (sulfate anion) (highest) complex ions | 265 pm (median) 229 pm (hexaaquanickel(II) cation) (lowest) 342 pm (hexaiodoplatinate(IV) anion) (highest) metal halide ion | 321 pm (median) 244 pm (hexafluorocobaltate(IV) anion) (lowest) 396 pm (hexaiodostannate(IV) anion) (highest) palladium 4 ion | 319 pm