Input interpretation

calcium chloride + pearl ash ⟶ potassium chloride + calcium carbonate
Balanced equation
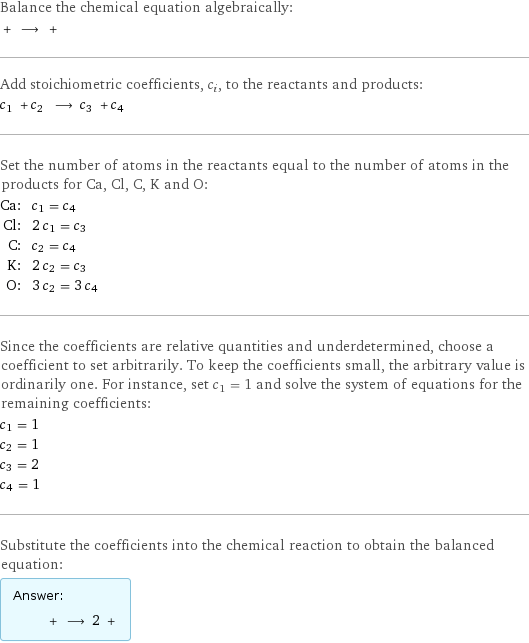
Balance the chemical equation algebraically: + ⟶ + Add stoichiometric coefficients, c_i, to the reactants and products: c_1 + c_2 ⟶ c_3 + c_4 Set the number of atoms in the reactants equal to the number of atoms in the products for Ca, Cl, C, K and O: Ca: | c_1 = c_4 Cl: | 2 c_1 = c_3 C: | c_2 = c_4 K: | 2 c_2 = c_3 O: | 3 c_2 = 3 c_4 Since the coefficients are relative quantities and underdetermined, choose a coefficient to set arbitrarily. To keep the coefficients small, the arbitrary value is ordinarily one. For instance, set c_1 = 1 and solve the system of equations for the remaining coefficients: c_1 = 1 c_2 = 1 c_3 = 2 c_4 = 1 Substitute the coefficients into the chemical reaction to obtain the balanced equation: Answer: | | + ⟶ 2 +
Structures
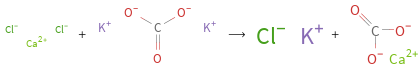
+ ⟶ +
Names
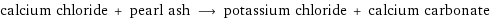
calcium chloride + pearl ash ⟶ potassium chloride + calcium carbonate
Reaction thermodynamics
Enthalpy
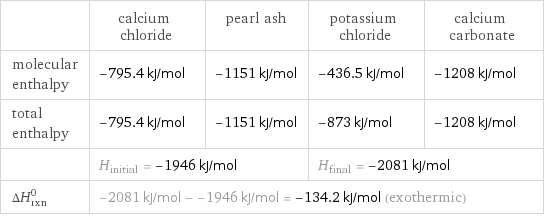
| calcium chloride | pearl ash | potassium chloride | calcium carbonate molecular enthalpy | -795.4 kJ/mol | -1151 kJ/mol | -436.5 kJ/mol | -1208 kJ/mol total enthalpy | -795.4 kJ/mol | -1151 kJ/mol | -873 kJ/mol | -1208 kJ/mol | H_initial = -1946 kJ/mol | | H_final = -2081 kJ/mol | ΔH_rxn^0 | -2081 kJ/mol - -1946 kJ/mol = -134.2 kJ/mol (exothermic) | | |
Gibbs free energy
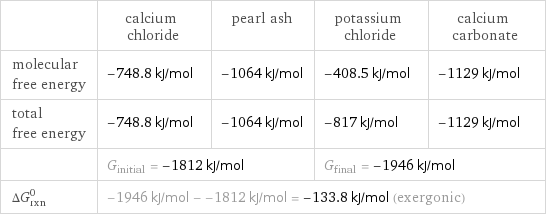
| calcium chloride | pearl ash | potassium chloride | calcium carbonate molecular free energy | -748.8 kJ/mol | -1064 kJ/mol | -408.5 kJ/mol | -1129 kJ/mol total free energy | -748.8 kJ/mol | -1064 kJ/mol | -817 kJ/mol | -1129 kJ/mol | G_initial = -1812 kJ/mol | | G_final = -1946 kJ/mol | ΔG_rxn^0 | -1946 kJ/mol - -1812 kJ/mol = -133.8 kJ/mol (exergonic) | | |
Chemical names and formulas
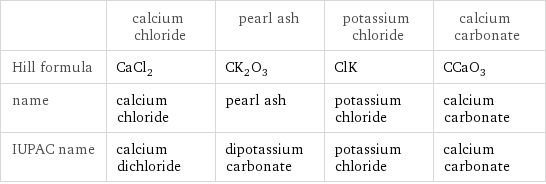
| calcium chloride | pearl ash | potassium chloride | calcium carbonate Hill formula | CaCl_2 | CK_2O_3 | ClK | CCaO_3 name | calcium chloride | pearl ash | potassium chloride | calcium carbonate IUPAC name | calcium dichloride | dipotassium carbonate | potassium chloride | calcium carbonate
Substance properties
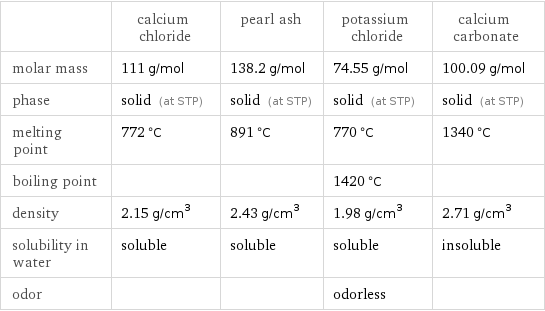
| calcium chloride | pearl ash | potassium chloride | calcium carbonate molar mass | 111 g/mol | 138.2 g/mol | 74.55 g/mol | 100.09 g/mol phase | solid (at STP) | solid (at STP) | solid (at STP) | solid (at STP) melting point | 772 °C | 891 °C | 770 °C | 1340 °C boiling point | | | 1420 °C | density | 2.15 g/cm^3 | 2.43 g/cm^3 | 1.98 g/cm^3 | 2.71 g/cm^3 solubility in water | soluble | soluble | soluble | insoluble odor | | | odorless |
Units
