Input interpretation
citric acid | hydrogen fluoride
Chemical names and formulas
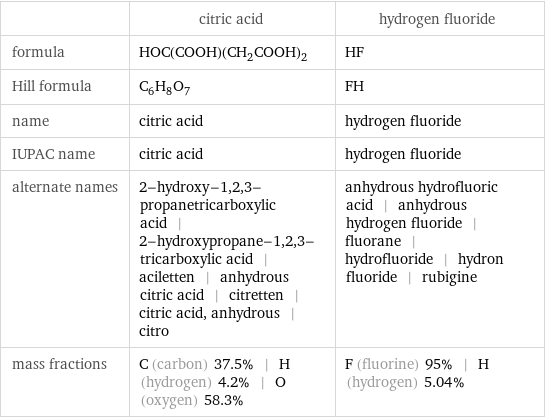
| citric acid | hydrogen fluoride formula | HOC(COOH)(CH_2COOH)_2 | HF Hill formula | C_6H_8O_7 | FH name | citric acid | hydrogen fluoride IUPAC name | citric acid | hydrogen fluoride alternate names | 2-hydroxy-1, 2, 3-propanetricarboxylic acid | 2-hydroxypropane-1, 2, 3-tricarboxylic acid | aciletten | anhydrous citric acid | citretten | citric acid, anhydrous | citro | anhydrous hydrofluoric acid | anhydrous hydrogen fluoride | fluorane | hydrofluoride | hydron fluoride | rubigine mass fractions | C (carbon) 37.5% | H (hydrogen) 4.2% | O (oxygen) 58.3% | F (fluorine) 95% | H (hydrogen) 5.04%
Structure diagrams
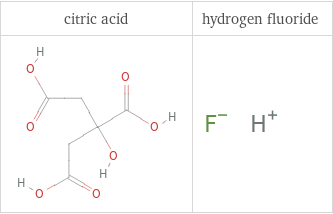
Structure diagrams
3D structure
3D structure
Basic properties
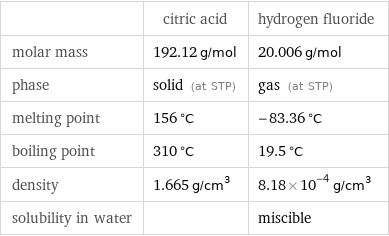
| citric acid | hydrogen fluoride molar mass | 192.12 g/mol | 20.006 g/mol phase | solid (at STP) | gas (at STP) melting point | 156 °C | -83.36 °C boiling point | 310 °C | 19.5 °C density | 1.665 g/cm^3 | 8.18×10^-4 g/cm^3 solubility in water | | miscible
Units

Hydrophobicity and permeability properties

| citric acid predicted LogP hydrophobicity | -1.33 experimental LogS | 0.51 predicted LogS | -0.26
Gas properties
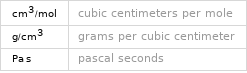
| hydrogen fluoride density | 8.18×10^-4 g/cm^3 vapor density | 1.27 molar volume | 24500 cm^3/mol refractive index | 1.00001 dynamic viscosity | 1.2571×10^-5 Pa s
Units
Thermodynamic properties
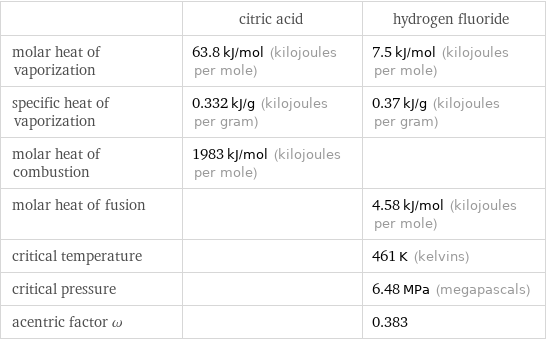
| citric acid | hydrogen fluoride molar heat of vaporization | 63.8 kJ/mol (kilojoules per mole) | 7.5 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.332 kJ/g (kilojoules per gram) | 0.37 kJ/g (kilojoules per gram) molar heat of combustion | 1983 kJ/mol (kilojoules per mole) | molar heat of fusion | | 4.58 kJ/mol (kilojoules per mole) critical temperature | | 461 K (kelvins) critical pressure | | 6.48 MPa (megapascals) acentric factor ω | | 0.383
Solid properties
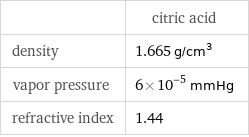
| citric acid density | 1.665 g/cm^3 vapor pressure | 6×10^-5 mmHg refractive index | 1.44
Units
