Input interpretation
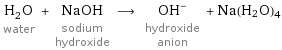
H_2O water + NaOH sodium hydroxide ⟶ (OH)^- hydroxide anion + Na(H2O)4
Chemical names and formulas
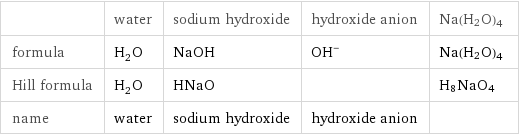
| water | sodium hydroxide | hydroxide anion | Na(H2O)4 formula | H_2O | NaOH | (OH)^- | Na(H2O)4 Hill formula | H_2O | HNaO | | H8NaO4 name | water | sodium hydroxide | hydroxide anion |
Substance properties
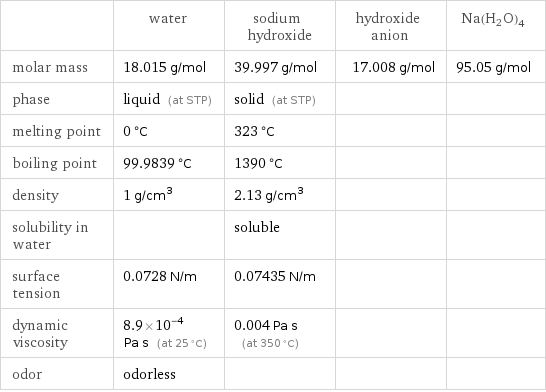
| water | sodium hydroxide | hydroxide anion | Na(H2O)4 molar mass | 18.015 g/mol | 39.997 g/mol | 17.008 g/mol | 95.05 g/mol phase | liquid (at STP) | solid (at STP) | | melting point | 0 °C | 323 °C | | boiling point | 99.9839 °C | 1390 °C | | density | 1 g/cm^3 | 2.13 g/cm^3 | | solubility in water | | soluble | | surface tension | 0.0728 N/m | 0.07435 N/m | | dynamic viscosity | 8.9×10^-4 Pa s (at 25 °C) | 0.004 Pa s (at 350 °C) | | odor | odorless | | |
Units
