Input interpretation
calcium sulfate
Chemical names and formulas
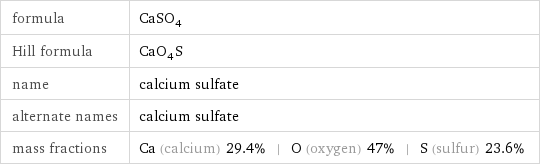
formula | CaSO_4 Hill formula | CaO_4S name | calcium sulfate alternate names | calcium sulfate mass fractions | Ca (calcium) 29.4% | O (oxygen) 47% | S (sulfur) 23.6%
Structure diagram
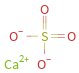
Structure diagram
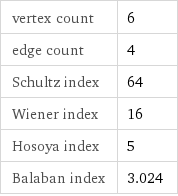
vertex count | 6 edge count | 4 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
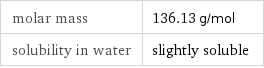
molar mass | 136.13 g/mol solubility in water | slightly soluble
Units

Thermodynamic properties
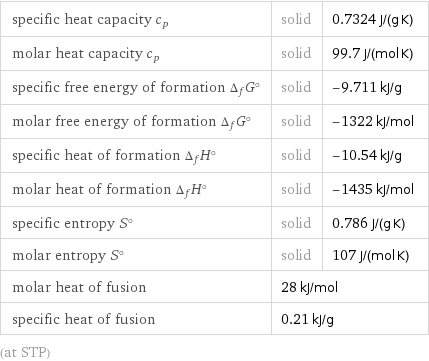
specific heat capacity c_p | solid | 0.7324 J/(g K) molar heat capacity c_p | solid | 99.7 J/(mol K) specific free energy of formation Δ_fG° | solid | -9.711 kJ/g molar free energy of formation Δ_fG° | solid | -1322 kJ/mol specific heat of formation Δ_fH° | solid | -10.54 kJ/g molar heat of formation Δ_fH° | solid | -1435 kJ/mol specific entropy S° | solid | 0.786 J/(g K) molar entropy S° | solid | 107 J/(mol K) molar heat of fusion | 28 kJ/mol | specific heat of fusion | 0.21 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7778-18-9 PubChem CID number | 24497 PubChem SID number | 24854355 SMILES identifier | [O-]S(=O)(=O)[O-].[Ca+2] InChI identifier | InChI=1/Ca.H2O4S/c;1-5(2, 3)4/h;(H2, 1, 2, 3, 4)/q+2;/p-2/fCa.O4S/qm;-2 RTECS number | WS6920000 MDL number | MFCD00010912](../image_source/9b8aba2d69b363474b0c11da52a128cc.png)
CAS number | 7778-18-9 PubChem CID number | 24497 PubChem SID number | 24854355 SMILES identifier | [O-]S(=O)(=O)[O-].[Ca+2] InChI identifier | InChI=1/Ca.H2O4S/c;1-5(2, 3)4/h;(H2, 1, 2, 3, 4)/q+2;/p-2/fCa.O4S/qm;-2 RTECS number | WS6920000 MDL number | MFCD00010912
Toxicity properties

odor | odorless
Ion equivalents

Ca^(2+) (calcium cation) | 1 (SO_4)^(2-) (sulfate anion) | 1