Input interpretation
germanium(IV) iodide
Chemical names and formulas
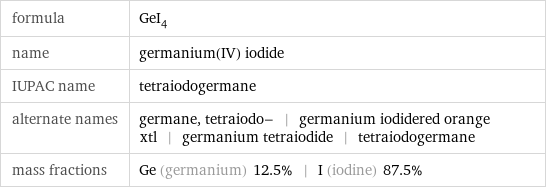
formula | GeI_4 name | germanium(IV) iodide IUPAC name | tetraiodogermane alternate names | germane, tetraiodo- | germanium iodidered orange xtl | germanium tetraiodide | tetraiodogermane mass fractions | Ge (germanium) 12.5% | I (iodine) 87.5%
Structure diagram

Structure diagram

| bond counts | bond lengths | bond energies | 4 bonds | 2.5 Å | 212 kJ/mol
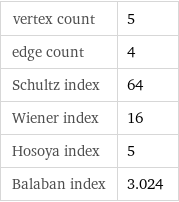
vertex count | 5 edge count | 4 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
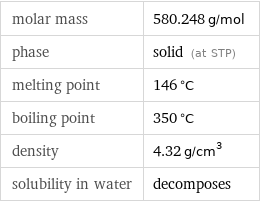
molar mass | 580.248 g/mol phase | solid (at STP) melting point | 146 °C boiling point | 350 °C density | 4.32 g/cm^3 solubility in water | decomposes
Units

Solid properties (at STP)

density | 4.32 g/cm^3 vapor pressure | 1.6×10^-7 mmHg (at 25 °C)
Units

Thermodynamic properties
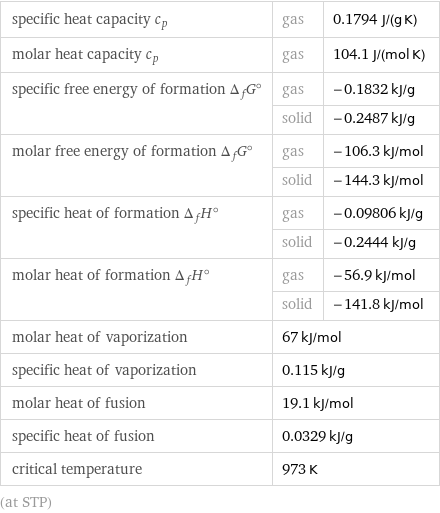
specific heat capacity c_p | gas | 0.1794 J/(g K) molar heat capacity c_p | gas | 104.1 J/(mol K) specific free energy of formation Δ_fG° | gas | -0.1832 kJ/g | solid | -0.2487 kJ/g molar free energy of formation Δ_fG° | gas | -106.3 kJ/mol | solid | -144.3 kJ/mol specific heat of formation Δ_fH° | gas | -0.09806 kJ/g | solid | -0.2444 kJ/g molar heat of formation Δ_fH° | gas | -56.9 kJ/mol | solid | -141.8 kJ/mol molar heat of vaporization | 67 kJ/mol | specific heat of vaporization | 0.115 kJ/g | molar heat of fusion | 19.1 kJ/mol | specific heat of fusion | 0.0329 kJ/g | critical temperature | 973 K | (at STP)
Chemical identifiers
(I)(I)I InChI identifier | InChI=1/GeI4/c2-1(3, 4)5 MDL number | MFCD00016120](../image_source/50040bba69222d6ca3aa5c1b9d65e962.png)
CAS number | 13450-95-8 PubChem CID number | 83479 PubChem SID number | 24863947 SMILES identifier | [Ge](I)(I)(I)I InChI identifier | InChI=1/GeI4/c2-1(3, 4)5 MDL number | MFCD00016120