Input interpretation
zirconium(II) chloride | hexafluorophosphoric acid
Chemical names and formulas
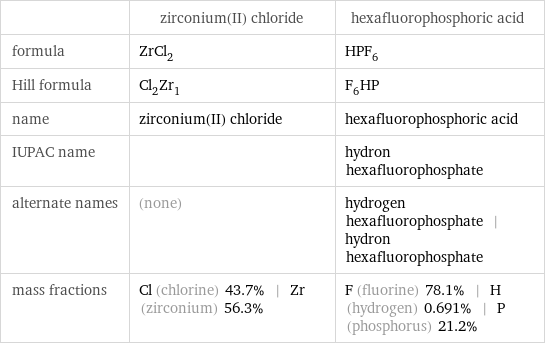
| zirconium(II) chloride | hexafluorophosphoric acid formula | ZrCl_2 | HPF_6 Hill formula | Cl_2Zr_1 | F_6HP name | zirconium(II) chloride | hexafluorophosphoric acid IUPAC name | | hydron hexafluorophosphate alternate names | (none) | hydrogen hexafluorophosphate | hydron hexafluorophosphate mass fractions | Cl (chlorine) 43.7% | Zr (zirconium) 56.3% | F (fluorine) 78.1% | H (hydrogen) 0.691% | P (phosphorus) 21.2%
Structure diagrams
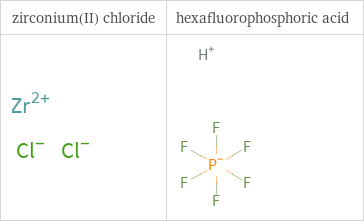
Structure diagrams
Basic properties
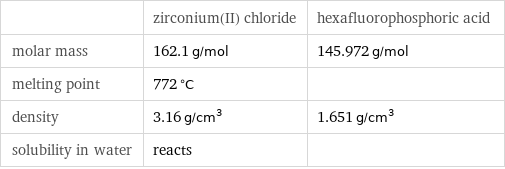
| zirconium(II) chloride | hexafluorophosphoric acid molar mass | 162.1 g/mol | 145.972 g/mol melting point | 772 °C | density | 3.16 g/cm^3 | 1.651 g/cm^3 solubility in water | reacts |
Units
