Input interpretation
aluminum iodide | gallium(III) perchlorate hydrate
Chemical names and formulas
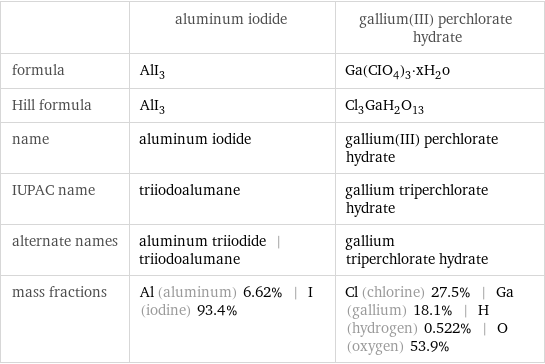
| aluminum iodide | gallium(III) perchlorate hydrate formula | AlI_3 | Ga(CIO_4)_3·xH_2o Hill formula | AlI_3 | Cl_3GaH_2O_13 name | aluminum iodide | gallium(III) perchlorate hydrate IUPAC name | triiodoalumane | gallium triperchlorate hydrate alternate names | aluminum triiodide | triiodoalumane | gallium triperchlorate hydrate mass fractions | Al (aluminum) 6.62% | I (iodine) 93.4% | Cl (chlorine) 27.5% | Ga (gallium) 18.1% | H (hydrogen) 0.522% | O (oxygen) 53.9%
Structure diagrams
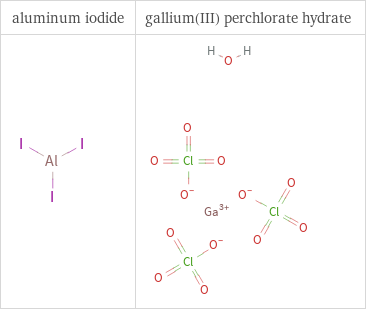
Structure diagrams
Basic properties
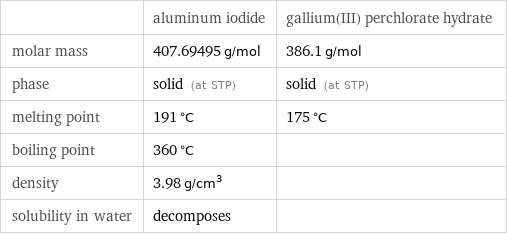
| aluminum iodide | gallium(III) perchlorate hydrate molar mass | 407.69495 g/mol | 386.1 g/mol phase | solid (at STP) | solid (at STP) melting point | 191 °C | 175 °C boiling point | 360 °C | density | 3.98 g/cm^3 | solubility in water | decomposes |
Units
