Input interpretation

insulators | first molar ionization energy
Summary
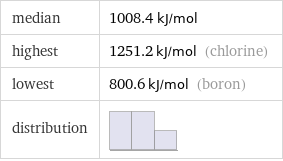
median | 1008.4 kJ/mol highest | 1251.2 kJ/mol (chlorine) lowest | 800.6 kJ/mol (boron) distribution |
Units

Ranked values
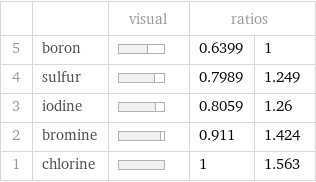
| | visual | ratios | 5 | boron | | 0.6399 | 1 4 | sulfur | | 0.7989 | 1.249 3 | iodine | | 0.8059 | 1.26 2 | bromine | | 0.911 | 1.424 1 | chlorine | | 1 | 1.563
First molar ionization energy rankings
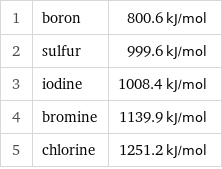
1 | boron | 800.6 kJ/mol 2 | sulfur | 999.6 kJ/mol 3 | iodine | 1008.4 kJ/mol 4 | bromine | 1139.9 kJ/mol 5 | chlorine | 1251.2 kJ/mol
Unit conversions for median first molar ionization energy 1008.4 kJ/mol

1.0084 MJ/mol (megajoules per mole)
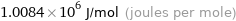
1.0084×10^6 J/mol (joules per mole)

241.01 kcal/mol (thermochemical kilocalories per mole)

10.451 eV (molar electronvolts)
Comparisons for median first molar ionization energy 1008.4 kJ/mol

≈ 0.43 × molar energy required to remove the first electron from helium ( 2372 kJ/mol )
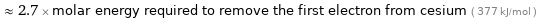
≈ 2.7 × molar energy required to remove the first electron from cesium ( 377 kJ/mol )