Input interpretation

period 4 elements | melting point
Summary
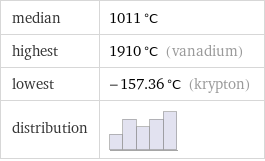
median | 1011 °C highest | 1910 °C (vanadium) lowest | -157.36 °C (krypton) distribution |
Plot
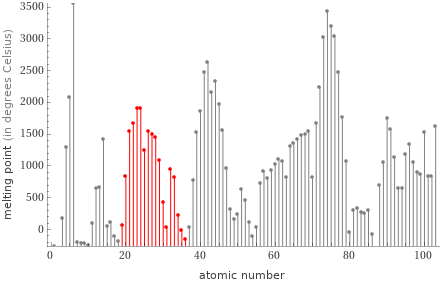
Plot
Distribution plots
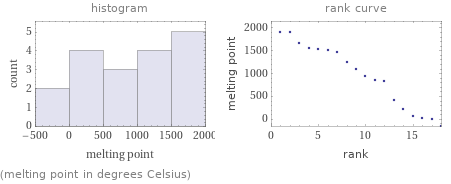
(melting point in degrees Celsius)
Melting point rankings
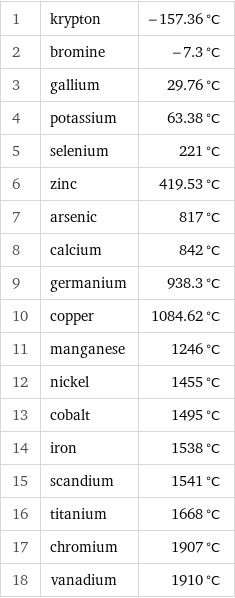
1 | krypton | -157.36 °C 2 | bromine | -7.3 °C 3 | gallium | 29.76 °C 4 | potassium | 63.38 °C 5 | selenium | 221 °C 6 | zinc | 419.53 °C 7 | arsenic | 817 °C 8 | calcium | 842 °C 9 | germanium | 938.3 °C 10 | copper | 1084.62 °C 11 | manganese | 1246 °C 12 | nickel | 1455 °C 13 | cobalt | 1495 °C 14 | iron | 1538 °C 15 | scandium | 1541 °C 16 | titanium | 1668 °C 17 | chromium | 1907 °C 18 | vanadium | 1910 °C
Unit conversions for median melting point 1011 °C
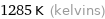
1285 K (kelvins)

1853 °F (degrees Fahrenheit)
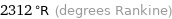
2312 °R (degrees Rankine)
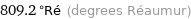
809.2 °Ré (degrees Réaumur)

538.5 °Rø (degrees Rømer)
Comparison for median melting point 1011 °C
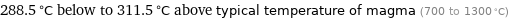
288.5 °C below to 311.5 °C above typical temperature of magma (700 to 1300 °C)
110 °C above large log fire temperature (1170 K)
Blackbody information for median melting point 1011 °C (degrees Celsius)
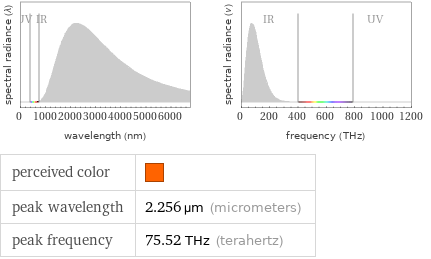
perceived color | peak wavelength | 2.256 µm (micrometers) peak frequency | 75.52 THz (terahertz)
Corresponding quantities
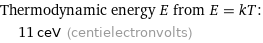
Thermodynamic energy E from E = kT: | 11 ceV (centielectronvolts)
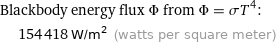
Blackbody energy flux Φ from Φ = σT^4: | 154418 W/m^2 (watts per square meter)
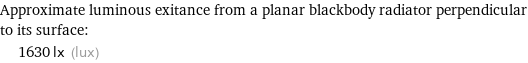
Approximate luminous exitance from a planar blackbody radiator perpendicular to its surface: | 1630 lx (lux)
Thermodynamic properties
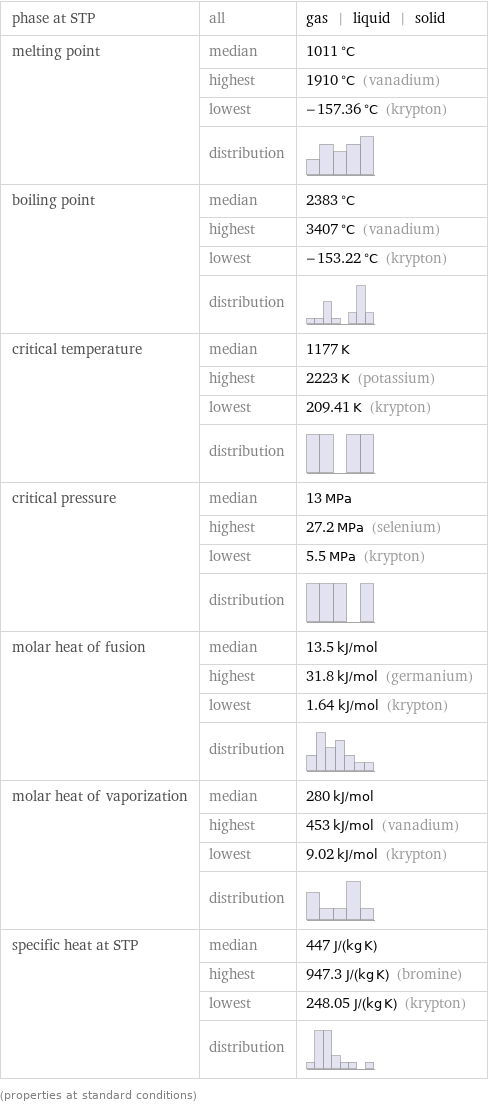
phase at STP | all | gas | liquid | solid melting point | median | 1011 °C | highest | 1910 °C (vanadium) | lowest | -157.36 °C (krypton) | distribution | boiling point | median | 2383 °C | highest | 3407 °C (vanadium) | lowest | -153.22 °C (krypton) | distribution | critical temperature | median | 1177 K | highest | 2223 K (potassium) | lowest | 209.41 K (krypton) | distribution | critical pressure | median | 13 MPa | highest | 27.2 MPa (selenium) | lowest | 5.5 MPa (krypton) | distribution | molar heat of fusion | median | 13.5 kJ/mol | highest | 31.8 kJ/mol (germanium) | lowest | 1.64 kJ/mol (krypton) | distribution | molar heat of vaporization | median | 280 kJ/mol | highest | 453 kJ/mol (vanadium) | lowest | 9.02 kJ/mol (krypton) | distribution | specific heat at STP | median | 447 J/(kg K) | highest | 947.3 J/(kg K) (bromine) | lowest | 248.05 J/(kg K) (krypton) | distribution | (properties at standard conditions)