Input interpretation
nickel(II) chloride | sodium chloride
Chemical names and formulas
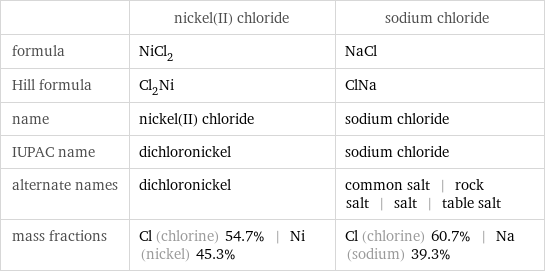
| nickel(II) chloride | sodium chloride formula | NiCl_2 | NaCl Hill formula | Cl_2Ni | ClNa name | nickel(II) chloride | sodium chloride IUPAC name | dichloronickel | sodium chloride alternate names | dichloronickel | common salt | rock salt | salt | table salt mass fractions | Cl (chlorine) 54.7% | Ni (nickel) 45.3% | Cl (chlorine) 60.7% | Na (sodium) 39.3%
Structure diagrams

Structure diagrams
Basic properties
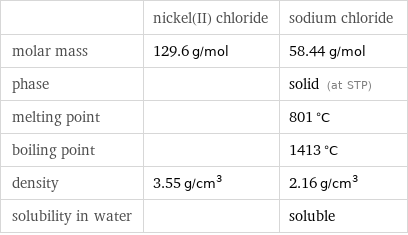
| nickel(II) chloride | sodium chloride molar mass | 129.6 g/mol | 58.44 g/mol phase | | solid (at STP) melting point | | 801 °C boiling point | | 1413 °C density | 3.55 g/cm^3 | 2.16 g/cm^3 solubility in water | | soluble
Units

Thermodynamic properties

| nickel(II) chloride | sodium chloride specific heat of fusion | 0.601 kJ/g (kilojoules per gram) | 0.4819 kJ/g (kilojoules per gram)
Solid properties
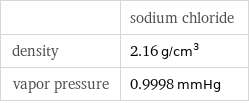
| sodium chloride density | 2.16 g/cm^3 vapor pressure | 0.9998 mmHg
Units
