Input interpretation

lead(II) zirconate | mercury
Chemical names and formulas
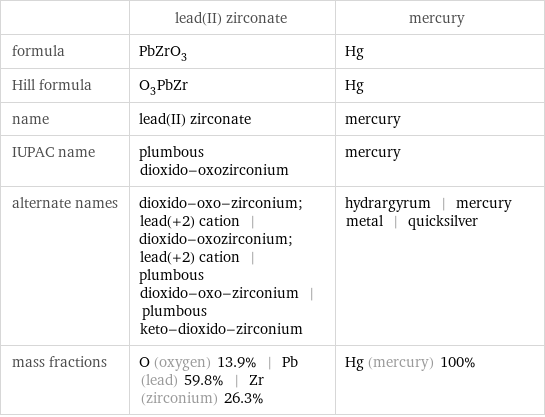
| lead(II) zirconate | mercury formula | PbZrO_3 | Hg Hill formula | O_3PbZr | Hg name | lead(II) zirconate | mercury IUPAC name | plumbous dioxido-oxozirconium | mercury alternate names | dioxido-oxo-zirconium; lead(+2) cation | dioxido-oxozirconium; lead(+2) cation | plumbous dioxido-oxo-zirconium | plumbous keto-dioxido-zirconium | hydrargyrum | mercury metal | quicksilver mass fractions | O (oxygen) 13.9% | Pb (lead) 59.8% | Zr (zirconium) 26.3% | Hg (mercury) 100%
Structure diagrams
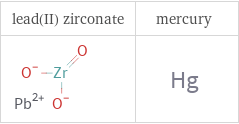
Structure diagrams
Basic properties
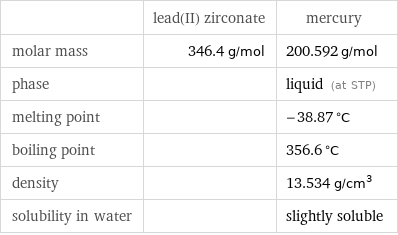
| lead(II) zirconate | mercury molar mass | 346.4 g/mol | 200.592 g/mol phase | | liquid (at STP) melting point | | -38.87 °C boiling point | | 356.6 °C density | | 13.534 g/cm^3 solubility in water | | slightly soluble
Units

Liquid properties
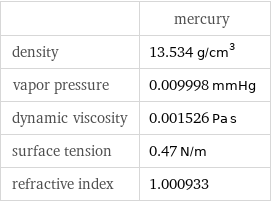
| mercury density | 13.534 g/cm^3 vapor pressure | 0.009998 mmHg dynamic viscosity | 0.001526 Pa s surface tension | 0.47 N/m refractive index | 1.000933
Units