Input interpretation
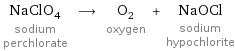
NaClO_4 sodium perchlorate ⟶ O_2 oxygen + NaOCl sodium hypochlorite
Balanced equation

Balance the chemical equation algebraically: NaClO_4 ⟶ O_2 + NaOCl Add stoichiometric coefficients, c_i, to the reactants and products: c_1 NaClO_4 ⟶ c_2 O_2 + c_3 NaOCl Set the number of atoms in the reactants equal to the number of atoms in the products for Cl, Na and O: Cl: | c_1 = c_3 Na: | c_1 = c_3 O: | 4 c_1 = 2 c_2 + c_3 Since the coefficients are relative quantities and underdetermined, choose a coefficient to set arbitrarily. To keep the coefficients small, the arbitrary value is ordinarily one. For instance, set c_1 = 1 and solve the system of equations for the remaining coefficients: c_1 = 1 c_2 = 3/2 c_3 = 1 Multiply by the least common denominator, 2, to eliminate fractional coefficients: c_1 = 2 c_2 = 3 c_3 = 2 Substitute the coefficients into the chemical reaction to obtain the balanced equation: Answer: | | 2 NaClO_4 ⟶ 3 O_2 + 2 NaOCl
Structures
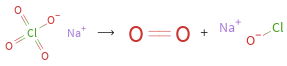
⟶ +
Names
sodium perchlorate ⟶ oxygen + sodium hypochlorite
Equilibrium constant
![Construct the equilibrium constant, K, expression for: NaClO_4 ⟶ O_2 + NaOCl Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the activity expression for each chemical species. • Use the activity expressions to build the equilibrium constant expression. Write the balanced chemical equation: 2 NaClO_4 ⟶ 3 O_2 + 2 NaOCl Assign stoichiometric numbers, ν_i, using the stoichiometric coefficients, c_i, from the balanced chemical equation in the following manner: ν_i = -c_i for reactants and ν_i = c_i for products: chemical species | c_i | ν_i NaClO_4 | 2 | -2 O_2 | 3 | 3 NaOCl | 2 | 2 Assemble the activity expressions accounting for the state of matter and ν_i: chemical species | c_i | ν_i | activity expression NaClO_4 | 2 | -2 | ([NaClO4])^(-2) O_2 | 3 | 3 | ([O2])^3 NaOCl | 2 | 2 | ([NaOCl])^2 The equilibrium constant symbol in the concentration basis is: K_c Mulitply the activity expressions to arrive at the K_c expression: Answer: | | K_c = ([NaClO4])^(-2) ([O2])^3 ([NaOCl])^2 = (([O2])^3 ([NaOCl])^2)/([NaClO4])^2](../image_source/17d579bf8a158faa66423df967678fad.png)
Construct the equilibrium constant, K, expression for: NaClO_4 ⟶ O_2 + NaOCl Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the activity expression for each chemical species. • Use the activity expressions to build the equilibrium constant expression. Write the balanced chemical equation: 2 NaClO_4 ⟶ 3 O_2 + 2 NaOCl Assign stoichiometric numbers, ν_i, using the stoichiometric coefficients, c_i, from the balanced chemical equation in the following manner: ν_i = -c_i for reactants and ν_i = c_i for products: chemical species | c_i | ν_i NaClO_4 | 2 | -2 O_2 | 3 | 3 NaOCl | 2 | 2 Assemble the activity expressions accounting for the state of matter and ν_i: chemical species | c_i | ν_i | activity expression NaClO_4 | 2 | -2 | ([NaClO4])^(-2) O_2 | 3 | 3 | ([O2])^3 NaOCl | 2 | 2 | ([NaOCl])^2 The equilibrium constant symbol in the concentration basis is: K_c Mulitply the activity expressions to arrive at the K_c expression: Answer: | | K_c = ([NaClO4])^(-2) ([O2])^3 ([NaOCl])^2 = (([O2])^3 ([NaOCl])^2)/([NaClO4])^2
Rate of reaction
![Construct the rate of reaction expression for: NaClO_4 ⟶ O_2 + NaOCl Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the rate term for each chemical species. • Write the rate of reaction expression. Write the balanced chemical equation: 2 NaClO_4 ⟶ 3 O_2 + 2 NaOCl Assign stoichiometric numbers, ν_i, using the stoichiometric coefficients, c_i, from the balanced chemical equation in the following manner: ν_i = -c_i for reactants and ν_i = c_i for products: chemical species | c_i | ν_i NaClO_4 | 2 | -2 O_2 | 3 | 3 NaOCl | 2 | 2 The rate term for each chemical species, B_i, is 1/ν_i(Δ[B_i])/(Δt) where [B_i] is the amount concentration and t is time: chemical species | c_i | ν_i | rate term NaClO_4 | 2 | -2 | -1/2 (Δ[NaClO4])/(Δt) O_2 | 3 | 3 | 1/3 (Δ[O2])/(Δt) NaOCl | 2 | 2 | 1/2 (Δ[NaOCl])/(Δt) (for infinitesimal rate of change, replace Δ with d) Set the rate terms equal to each other to arrive at the rate expression: Answer: | | rate = -1/2 (Δ[NaClO4])/(Δt) = 1/3 (Δ[O2])/(Δt) = 1/2 (Δ[NaOCl])/(Δt) (assuming constant volume and no accumulation of intermediates or side products)](../image_source/9dfcb97d01e0e6c94cc1248bf6d3aa01.png)
Construct the rate of reaction expression for: NaClO_4 ⟶ O_2 + NaOCl Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the rate term for each chemical species. • Write the rate of reaction expression. Write the balanced chemical equation: 2 NaClO_4 ⟶ 3 O_2 + 2 NaOCl Assign stoichiometric numbers, ν_i, using the stoichiometric coefficients, c_i, from the balanced chemical equation in the following manner: ν_i = -c_i for reactants and ν_i = c_i for products: chemical species | c_i | ν_i NaClO_4 | 2 | -2 O_2 | 3 | 3 NaOCl | 2 | 2 The rate term for each chemical species, B_i, is 1/ν_i(Δ[B_i])/(Δt) where [B_i] is the amount concentration and t is time: chemical species | c_i | ν_i | rate term NaClO_4 | 2 | -2 | -1/2 (Δ[NaClO4])/(Δt) O_2 | 3 | 3 | 1/3 (Δ[O2])/(Δt) NaOCl | 2 | 2 | 1/2 (Δ[NaOCl])/(Δt) (for infinitesimal rate of change, replace Δ with d) Set the rate terms equal to each other to arrive at the rate expression: Answer: | | rate = -1/2 (Δ[NaClO4])/(Δt) = 1/3 (Δ[O2])/(Δt) = 1/2 (Δ[NaOCl])/(Δt) (assuming constant volume and no accumulation of intermediates or side products)
Chemical names and formulas
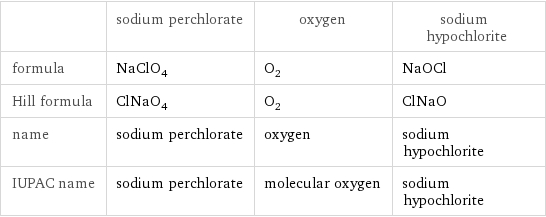
| sodium perchlorate | oxygen | sodium hypochlorite formula | NaClO_4 | O_2 | NaOCl Hill formula | ClNaO_4 | O_2 | ClNaO name | sodium perchlorate | oxygen | sodium hypochlorite IUPAC name | sodium perchlorate | molecular oxygen | sodium hypochlorite
Substance properties
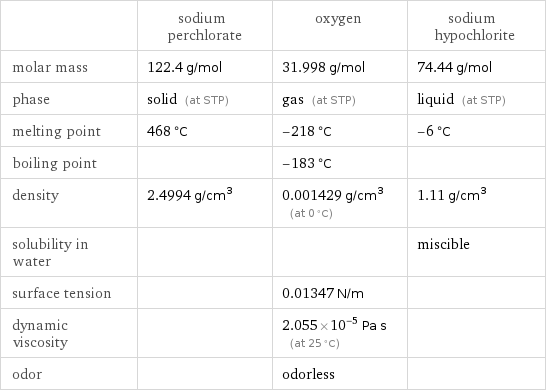
| sodium perchlorate | oxygen | sodium hypochlorite molar mass | 122.4 g/mol | 31.998 g/mol | 74.44 g/mol phase | solid (at STP) | gas (at STP) | liquid (at STP) melting point | 468 °C | -218 °C | -6 °C boiling point | | -183 °C | density | 2.4994 g/cm^3 | 0.001429 g/cm^3 (at 0 °C) | 1.11 g/cm^3 solubility in water | | | miscible surface tension | | 0.01347 N/m | dynamic viscosity | | 2.055×10^-5 Pa s (at 25 °C) | odor | | odorless |
Units
