Input interpretation
magnesium chloride | lithium hydroxide
Chemical names and formulas
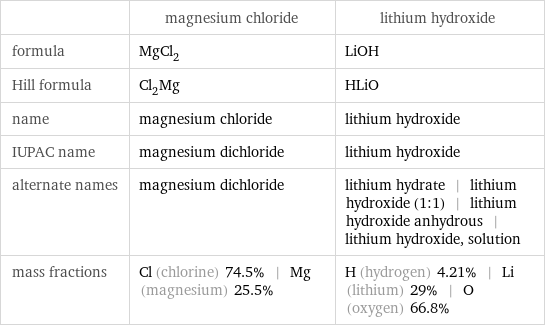
| magnesium chloride | lithium hydroxide formula | MgCl_2 | LiOH Hill formula | Cl_2Mg | HLiO name | magnesium chloride | lithium hydroxide IUPAC name | magnesium dichloride | lithium hydroxide alternate names | magnesium dichloride | lithium hydrate | lithium hydroxide (1:1) | lithium hydroxide anhydrous | lithium hydroxide, solution mass fractions | Cl (chlorine) 74.5% | Mg (magnesium) 25.5% | H (hydrogen) 4.21% | Li (lithium) 29% | O (oxygen) 66.8%
Structure diagrams
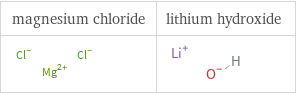
Structure diagrams
Basic properties
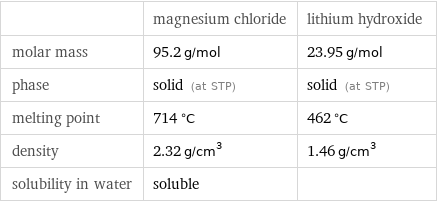
| magnesium chloride | lithium hydroxide molar mass | 95.2 g/mol | 23.95 g/mol phase | solid (at STP) | solid (at STP) melting point | 714 °C | 462 °C density | 2.32 g/cm^3 | 1.46 g/cm^3 solubility in water | soluble |
Units

Thermodynamic properties
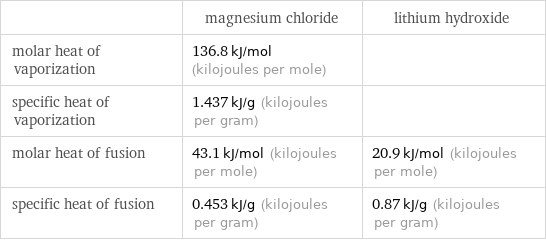
| magnesium chloride | lithium hydroxide molar heat of vaporization | 136.8 kJ/mol (kilojoules per mole) | specific heat of vaporization | 1.437 kJ/g (kilojoules per gram) | molar heat of fusion | 43.1 kJ/mol (kilojoules per mole) | 20.9 kJ/mol (kilojoules per mole) specific heat of fusion | 0.453 kJ/g (kilojoules per gram) | 0.87 kJ/g (kilojoules per gram)
Solid properties (at STP)

| magnesium chloride | lithium hydroxide density | 2.32 g/cm^3 | 1.46 g/cm^3 vapor pressure | | 2 mmHg
Units

Chemical identifiers
![| magnesium chloride | lithium hydroxide CAS number | 7786-30-3 | 1310-65-2 PubChem CID number | 24584 | 3939 PubChem SID number | 24852507 | SMILES identifier | [Mg+2].[Cl-].[Cl-] | [Li+].[OH-]](../image_source/b0b63adb5f2d879e56e77490f156753f.png)
| magnesium chloride | lithium hydroxide CAS number | 7786-30-3 | 1310-65-2 PubChem CID number | 24584 | 3939 PubChem SID number | 24852507 | SMILES identifier | [Mg+2].[Cl-].[Cl-] | [Li+].[OH-]