Input interpretation
silver bromate
Chemical names and formulas

formula | AgBrO_3 name | silver bromate alternate names | bromic acid; silver | silver(1) bromate mass fractions | Ag (silver) 45.8% | Br (bromine) 33.9% | O (oxygen) 20.4%
Structure diagram
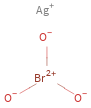
Structure diagram
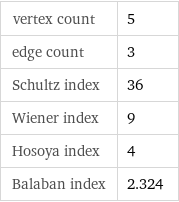
vertex count | 5 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties
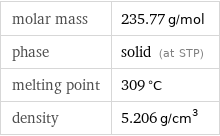
molar mass | 235.77 g/mol phase | solid (at STP) melting point | 309 °C density | 5.206 g/cm^3
Units

Solid properties (at STP)

density | 5.206 g/cm^3
Units

Thermodynamic properties
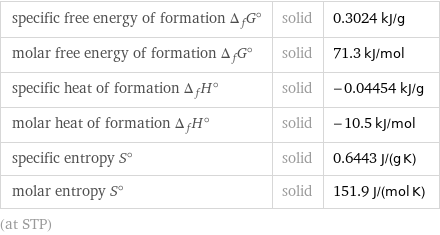
specific free energy of formation Δ_fG° | solid | 0.3024 kJ/g molar free energy of formation Δ_fG° | solid | 71.3 kJ/mol specific heat of formation Δ_fH° | solid | -0.04454 kJ/g molar heat of formation Δ_fH° | solid | -10.5 kJ/mol specific entropy S° | solid | 0.6443 J/(g K) molar entropy S° | solid | 151.9 J/(mol K) (at STP)
Chemical identifiers
![CAS number | 7783-89-3 SMILES identifier | [Ag+].Br(=O)(=O)[O-] MDL number | MFCD00046157](../image_source/d93807a72d67d4f43d915814d9652f11.png)
CAS number | 7783-89-3 SMILES identifier | [Ag+].Br(=O)(=O)[O-] MDL number | MFCD00046157
NFPA label

NFPA label

NFPA hazards | oxidizing agent
Ion equivalents

Ag^+ (silver(I) cation) | 1 (BrO_3)^- (bromate anion) | 1