Input interpretation
sodium chloride | elemental oxidation states
Result
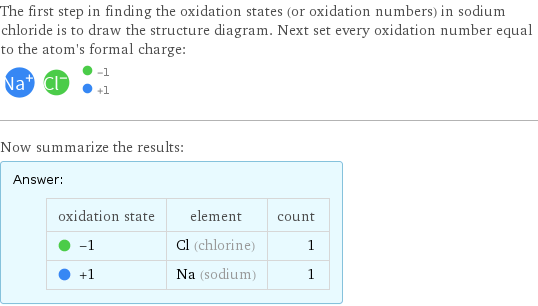
The first step in finding the oxidation states (or oxidation numbers) in sodium chloride is to draw the structure diagram. Next set every oxidation number equal to the atom's formal charge: Now summarize the results: Answer: | | oxidation state | element | count -1 | Cl (chlorine) | 1 +1 | Na (sodium) | 1
Chemical names and formulas
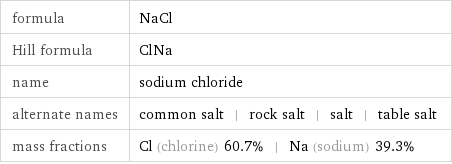
formula | NaCl Hill formula | ClNa name | sodium chloride alternate names | common salt | rock salt | salt | table salt mass fractions | Cl (chlorine) 60.7% | Na (sodium) 39.3%
Ion equivalents

Na^+ (sodium cation) | 1 Cl^- (chloride anion) | 1