Input interpretation
period 6 elements | number of valence electrons
Summary
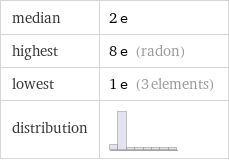
median | 2 e highest | 8 e (radon) lowest | 1 e (3 elements) distribution |
Units

Number of valence electrons rankings
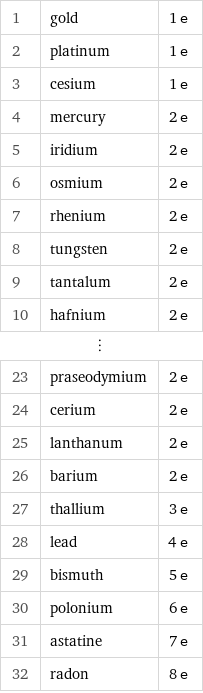
1 | gold | 1 e 2 | platinum | 1 e 3 | cesium | 1 e 4 | mercury | 2 e 5 | iridium | 2 e 6 | osmium | 2 e 7 | rhenium | 2 e 8 | tungsten | 2 e 9 | tantalum | 2 e 10 | hafnium | 2 e ⋮ | | 23 | praseodymium | 2 e 24 | cerium | 2 e 25 | lanthanum | 2 e 26 | barium | 2 e 27 | thallium | 3 e 28 | lead | 4 e 29 | bismuth | 5 e 30 | polonium | 6 e 31 | astatine | 7 e 32 | radon | 8 e
Corresponding quantity
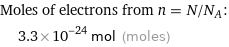
Moles of electrons from n = N/N_A: | 3.3×10^-24 mol (moles)